Abstract
The prefrontal cortex (PFC) provides the structural basis for numerous higher cognitive functions. However, it is still largely unknown which mechanisms provide the functional basis for flexible cognitive control of goal-directed behavior. Here, we review recent findings, which suggest that the functional architecture of cognition is profoundly rhythmic and propose that the PFC serves as a conductor to orchestrate task-relevant large-scale networks. We highlight several studies that demonstrated that oscillatory dynamics, such as phase resetting, cross-frequency coupling and entrainment, support PFC-dependent recruitment of task-relevant regions into coherent functional networks. Importantly, these findings support the notion that distinct spectral signatures reflect different cortical computations supporting effective multiplexing on different temporal channels along the same anatomical pathways.
Keywords: Prefrontal cortex, neuronal oscillations, large-scale networks, cross-frequency coupling, network connectivity, neuronal entrainment
The functional architecture of cognition is rhythmic
Cognition and the executive control of goal-directed behavior are highly flexible and rapidly integrate task-relevant information according to the current contexts and demands. However, the neuronal basis of higher cognitive functions is still largely unknown. Results from numerous lesion studies have suggested that the prefrontal cortex is essential for the organization and control of goal-directed behavior [1]. In addition, various reports have emphasized the role of PFC activity patterns, thought to reflect goals and the means to achieve fluid behavior [2].
It has been argued that cognition might be the result of frequency-specific interactions of specialized but widely distributed cortical regions [3,4]. Importantly, this line of research accentuates the importance of rhythmic brain activity for coordination of large-scale cortical dynamics to support cognitive processing and goal-directed behavior [5]. It has also been demonstrated that neuronal oscillation have a causal role for perception and cognition [5,6] and do not constitute an epiphenomenon of spiking activity. Most of the oscillatory mechanisms were initially described in the hippocampus and primary sensory areas of rodents and non-human primates [7–11], but recent advances suggested that higher cognitive processing might employ similar network mechanisms.
Several studies have begun to demonstrate that cognitive processing exhibits rhythmic fluctuations, linking the oscillatory patterns of neuronal activity to periodic fluctuations in perception [12], attention [13–15], decision-making [16] or memory reactivation [17]. For example, it has been demonstrated that visual perception cycles as a function of the cortical alpha (8-12 Hz) phase [12]. In addition, recent reports have shown that the allocation of attention varies periodically as a function of low frequency oscillations [13–15]. Several novel non-invasive brain stimulation approaches, which allow for the frequency-specific entrainment of neuronal activity, have causally linked neuronal oscillations to perception and behavior [5,18,19]. In this review, we aim to integrate these diverse lines of research and review findings that endogenous neuronal oscillations provide the functional architecture of conscious perception and various higher cognitive functions.
Several behavioral findings have suggested that cognitive processing might be discrete and not continuous [20]. Thus, we first survey several oscillatory mechanisms that may guide goal-directed behavior and then discuss how oscillations form transient large-scale frequency-specific networks to support cognitive processing [3,21,22]. We focus mainly on intracranial electrophysiological studies in human and non-human primates, which provide an unprecedented spatiotemporal resolution to study cognition in the sub-millisecond and sub-centimeter range. Next, we discuss the functional organization across several spatial and temporal scales and how different oscillations might dynamically interact to enable cognitive control. Importantly, we review recent advances in analyzing non-linear neural dynamics that predict behavior on the single trial level. Finally, we highlight how different state-of-the-art methods can be utilized to fully characterize the structural and functional constitution of the fronto-parietal network. Thus, we propose that the PFC serves as a ‘conductor’ that rapidly integrates task-relevant information and orchestrates large-scale networks. We argue that oscillatory dynamics might support rapid activity-silent encoding and the selective modulation of activity in distant cortical sites. Taken together, this review posits that the functional architecture of cognition is innately rhythmic.
Oscillatory mechanisms guiding behavior and cognition
Classic models of cognitive processing, such as the drift diffusion model for decision-making or the persistent delay activity model of working memory (WM), emphasize the importance of activation of single neurons for effective cortical processing [23]. These models have recently been questioned by several studies [24–26]. For example, it has been suggested that sustained activity at the population level might reflect an artifact of averaging across multiple trials with different onset latencies of short lasting activity bursts [23]. While activation does not necessarily imply causation [27], several recent findings convincingly demonstrated that the exact timing of ensemble activity predicted behavior on the single trial level [28]. In this context, it has been argued that neuronal oscillations could provide a temporal reference frame to control cortical excitability and spike timing [6,7,29]. Traditionally, the local field potential (LFP) has been seen as an epiphenomenon of spiking activity [7], but recent findings revealed that narrow-banded oscillatory activity could reflect a feedback mechanism to control spiking activity [6] and coordinate neuronal ensembles to generate behavior [26]. These studies do not imply that single unit activity is not a central component of behavior, but rather suggest that oscillations triggered by single unit activity (SUA) activity are used in concert with SUA to shape behavior [26].
Findings from several studies indicate that much of the processing in the PFC is activity-silent [30]. For example, a human intracranial study measuring high frequency neural activity demonstrated that the PFC only became active when unpredicted deviants were detected (Figure 1A, [31]). Neither predicted deviants nor standards elicited any meaningful PFC activation, while sensory areas did not distinguish between predicted and unpredicted deviants. Notably, the PFC became active when unexpected errors were detected [32]. This raises the question how the PFC encodes predicted task contexts and other behaviorally relevant rules. Here, we argue that oscillatory dynamics on the level of large-scale networks support the activity-silent encoding of task-relevant contexts and rules (Figure 1B). We then discuss exemplary mechanisms (Figure 1C-F) of how endogenous oscillations might be used by the PFC to guide behavior.
Figure 1. Oscillatory mechanisms supporting cognitive processing in frontal cortex.
(A) High gamma responses to standard and deviants in sensory and frontal regions. Note, that only unpredicted deviants evoke a strong response in PFC, raising the questions of how predictions are implemented in frontal areas. (B) Illustration of two predicted contexts, where a brief burst of activity might be coordinated by the underlying oscillatory dynamics. Different contexts could be embedded in distinct spatiotemporal configurations (red letters indicate examples in Figure 1C-F) of the same network. Hence, the PFC only becomes active if a novel context is presented. (C) Phase resetting at the beginning of the trial is stronger for correct shifts in attention. The grey lines indicate the low frequency phase of single trials. Note the increased phase consistency for correct trials (upper panel). (D) Activity at different time points during the oscillatory cycle encodes distinct categories. Houses (blue), scenes (red), tools (green) and faces (black) were encoded at different phases and frequencies of the underlying low-frequency oscillation. (E) Cross-frequency coupling could mediate cortical computations and information integration across several temporal scales. The example data shows that the phase of delta/theta activity (2-5 Hz) modulates the amplitude in a broad range of frequencies (10–250 Hz). (F) Frequency-specific connectivity patterns encode distinct task relevant rules. The schematic depicts how the same neuronal assembly might have been differentially connected to encode two different rules (rule 1: color vs. rule 2: orientation, [41]). Furthermore, different frequency bands allow multiplexing different computations on several temporal scales. The graphs in a and c are reproduced with permission from [31,33]. The graphs in d and e appeared under the Creative Commons Attribution (CC BY) license [28,38].
Multiple studies have demonstrated that phase resetting of low frequency oscillations by task-relevant cues facilitates subsequent behavior [33–35]. For example, it has been shown that correct shifts in attention lead to pronounced phase resets in prefrontal and cingulate areas, which were absent on error trials (Figure 1C, [33]). The authors argued that phase resetting imposes coherent activity in wide-spread cortical regions aligning spatiotemporal dynamics in task-relevant sites. Hence, phase resetting could control the exact timing of neuronal activity, e.g. that a burst of activity coincides with the next behaviorally relevant event at a certain LFP phase to enable efficient cortical processing and inter-areal communication [33,34]. However, it is currently unclear if the observed theta phase resetting signatures in non-human primates generalize to humans, where the opposite has been reported [36,37].
The importance of oscillatory phase was further highlighted by the recent observation that distinct stimulus categories might be encoded at different phase angles of low frequency oscillations (Figure 1D, [38]). This finding was in line with the notion that activity at different phase angles supports temporal order in WM [39]. Interestingly both reports implied that this phase encoding is also associated with an increase in cross-frequency coupling (CFC, see Glossary, [38,39]). In particular, CFC might subserve the cortical organization across temporal scales (Figure 1E, [11,40]). In other words, activity at different phases of the ongoing activity might carry distinct behaviorally relevant information.
Finally, it has been suggested that phase synchronous ensembles form task-relevant networks, which coordinate intra- and inter-areal information flow. For example, it has been demonstrated that different rules are reflected in distinct synchronization patterns within prefrontal assemblies [41]. Next, we discuss PFC-dependent large-scale networks that support distinct cognitive processes and goal-directed behavior.
Large-scale networks dependent on prefrontal cortex
It has long been suspected that cognitive control and the means to achieve fluid goal-directed behavior stem from activity patterns in the PFC, which selectively bias neuronal activity in distant cortical and subcortical regions and which control the information flow in large-scale neuronal networks [2]. The popular communication-through-coherence (CTC) hypothesis suggested that neuronal communication might be established through coherently oscillating neuronal assemblies [4]. Over the last decade numerous studies investigated large-scale neuronal dynamics and the role of synchronous activity for cognition and behavior. However, most studies focused on cortico-cortical interactions [3]. In particular, the fronto-parietal network has been studied extensively and it has been suggested to constitute a core element of flexible cognitive control. Recent findings further supported the role of synchronous oscillatory activity for effective fronto-parieto-occipital [34,42–46] and fronto-temporal communication [47,48].
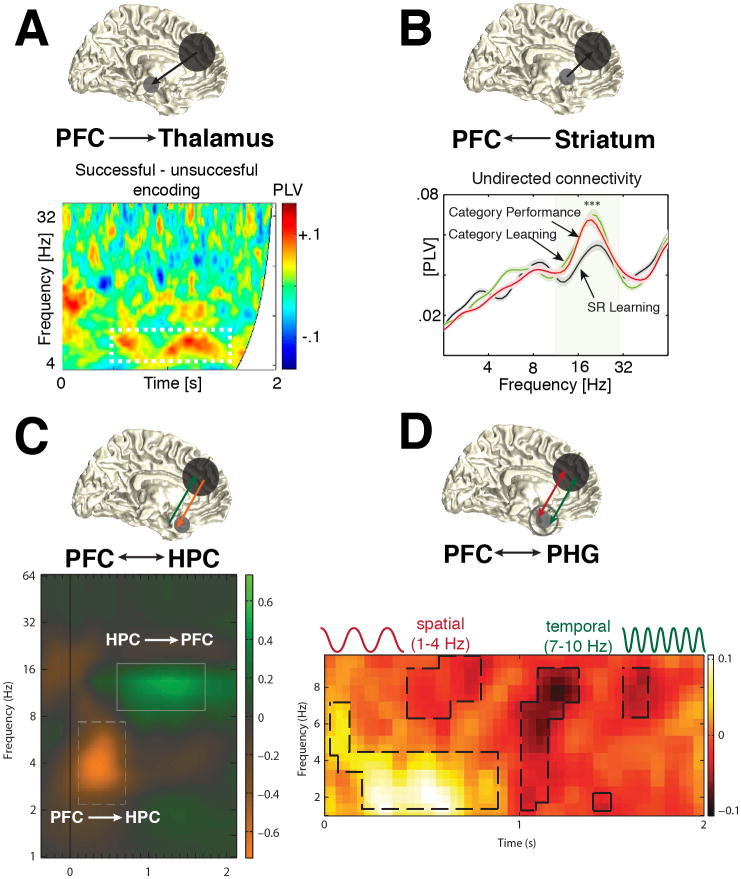
Here, we focus on prefrontal-subcortical interactions, which are less accessible by non-invasive approaches. For example, a recent intracranial study in humans investigated the role of phase synchrony between frontal areas and the anterior thalamic nucleus (ATN) for memory formation (Figure 2A, [49]). The authors found increased PFC-ATN phase-locking in the theta-band (4-8 Hz) for successfully encoded items. In general, theta oscillations have long been associated with memory formation and are most prominent in the MTL [35,50]. The role of prefrontal-hippocampal theta synchrony for memory integration has further been substantiated by recent MEG studies, which showed that stronger theta coherence between PFC and HPC predicted subsequent memory formation [51,52]. In addition, several studies also established a link between beta oscillations and memory formation [39,53,54]. A recent monkey study found that synchronous beta-band activity between the PFC and the striatum was stronger during category learning (Figure 2B, [54]). Interestingly, a related study revealed that both theta and alpha/beta synchrony were elevated during learning and recall (Figure 2C, [55]). A similar finding in humans also reported a dissociation of different frequency bands and attributed prefrontal-parahippocampal network connectivity in the delta/theta range (1-4 Hz) to successful spatial context retrieval, while increased theta/alpha synchrony (7-10 Hz) correlated with correct temporal context retrieval (Figure 2D, [56]).
Figure 2. Prefrontal cortex dependent large-scale networks.
(A) PFC-Thalamus: Increased phase-locking between frontal EEG sensors and the right anterior thalamic nucleus (RATN) for successfully encoded items. (B) PFC-Striatum: Undirected connectivity for category learning/performance and stimulus-response (SR) learning. Note the significant peak in the beta-band (around 20 Hz) for category over SR learning. Directional connectivity analyses between the PFC and striatum revealed that beta interactions signaled mainly the information flow from striatum to the PFC and not vice versa. (C) PFC-Hippocampus: Differences in inter-areal connectivity. While changes in the alpha-band reflected directional interactions from Hippocampus to the PFC, theta-band activity supported information flow in the opposite direction. (D) PFC-Parahippocampal gyrus: Network synchronization the delta (1-4 Hz) and theta/alpha-bands (7-10 Hz) multiplexed correct retrieval for spatial (delta) or temporal (theta/alpha) contexts. The graph in a appeared under the Creative Commons Attribution (CC BY) license [49]. The graphs in b-d are reproduced with permission from [54–56].
Taken together, these findings have been interpreted as evidence that the rich spatiotemporal correlation structure of the brain might enable effective cortical computation and information transfer [3,4,57]. This is in line with the spectral fingerprint hypothesis, which proposed that different spectral patterns might reflect distinct canonical neuronal computations [3]. Interestingly, most findings on inter-areal long-range connectivity highlighted a role of synchronized low frequency oscillations (< 30 Hz), while high frequency activity (> 30 Hz) probably reflects broadband shifts due to changes in the neuronal firing rate [58,59]. Recently, it has been argued that amplitude correlations of high frequency activity might capture interactions between functionally connected, but non-synchronous regions [57]. Currently, it is unclear whether phase- and amplitude-based connectivity metrics capture similar dynamics or whether they constitute independent modes of interaction [60,61].
Thus far, most of the presented evidence has been correlative in nature. Non-invasive brain stimulation approaches were recently used to causally probe the role of distinct spectral signatures for top-down processing [5,18]. For example, tACS was used to confirm the role of prefrontal theta activity [62] and long-range theta synchronization for WM [63]. In the future, network perturbation approaches will become more important to causally link synchronized neuronal oscillatory activity to perception and behavior [5,18].
Taken together, there is increasing evidence that the PFC constitutes a central hub, which flexibly interacts with task-relevant cortical sites to implement flexible cognitive control and goal-directed behavior. However, it is unclear how prefrontal ensembles are organized to integrate multiple endogenous priors with task-relevant cues to orchestrate subsequent behavior.
Multiplexed cognition and its spatiotemporal organization
Results from several single unit studies indicate that neuronal populations in the prefrontal cortex exhibit a mixed selectivity [64–68], i.e. these populations are able to engage in different tasks facilitating cognitive flexibility. However, it is unknown how these neuronal assemblies are recruited into an active circuit, while simultaneously providing feedback to downstream regions. It has been suggested that temporal multiplexing might constitute a key mechanism of prefrontal integrative functions [2,69,70]. Multiplexing refers to a process where different computations are carried out in distinct frequency-bands and thus, can successfully be separated on different temporal channels [69]. For example, it has been shown that spatial and temporal context retrieval is supported by the same anatomical network, which included prefrontal and MTL structures (Figure 2D, [56]). Crucially, the authors demonstrated that the exact frequency determined whether spatial or temporal context was recalled. Notably, a similar mechanism was observed for PFC-hippocampus interactions in the rats [71] and monkeys [55], where the exact frequency determined the directionality of the information flow. While directional PFC to HPC synchrony was implemented in the theta-band, feedback was provided in the alpha-/low-beta-band (Figure 2C). Interestingly, these spectral fingerprints changed during learning: While theta synchrony dropped after the initial learning, alpha synchrony increased. In other words, during the initial learning the information flowed mainly from PFC to the hippocampus, while the direction reversed in later learning stages. Multiplexing has also been observed in the visual system of both humans and monkeys [72,73]. Here, theta and gamma oscillations mediate feed-forward influences (from lower to higher visual areas), while alpha and low-beta oscillations provide top-down feedback. A recent lesion study in monkeys confirmed the top-down nature of beta oscillations, which were still present in extrastriate areas after removal of the primary visual cortex [74]. Taken together, there is increasing evidence that cognitive processing is simultaneously distributed across several spatiotemporal scales, raising the question how these distinct spectral signatures dynamically interact to enable effective cortical processing and communication [75].
Over the last decade, multiple findings suggested that different spectral signatures do not occur in isolation, but are functionally coupled through CFC [11]. It has been argued that CFC constitutes a key mechanism to coordinate the spatiotemporal organization of neuronal networks. Therefore, it has been proposed that regions that exhibit local CFC are also more likely to engage in inter-regional connectivity [35,60]. CFC captures these non-linear cortical dynamics, which might track behavior better than linear measures (Box 1). For example, a recent intracranial attention study in humans showed that delta-gamma CFC in frontal and parietal areas predicted reaction times on a trial-by-trial basis (Figure 3B, [28]). Recent tACS studies confirmed that cognitive control critically depends on both coordinated theta-gamma CFC in PFC [76] and PFC-PPC synchrony [63]. Notably, there is an ongoing debate about whether the low frequency phase drives the amplitude of the high frequency component or vice versa [77]. It has been suggested that both components could drive the interaction to facilitate information integration across temporal scales [78], but it is unclear how directional synchrony across several spatiotemporal scales is established.
Box 1. Pitfalls of analyzing non-linear dynamics in electrophysiological data.
Linear analysis techniques provide valuable insights into brain-behavior relationships. Recently, non-linear approaches assessing the phase of oscillatory brain activity have gained interest. For example, circular statistics [116] have been used to reveal periodicities in sensory or cognitive functions1 and address the CTC hypothesis [4] that postulates an important role of the oscillatory phase for inter-areal communication. Furthermore, CFC analyses demonstrate a systematic relationship between the phase of slower oscillations and the amplitude of high frequency activity [11,117]. Hence, CFC has been suggested to play an important role for the spatiotemporal organization of large-scale networks [11]. However, a number of pitfalls hamper the phase-dependent analyses [40]. In general, connectivity and CFC analyses are problematic if a single process affects multiple sensors (e.g. volume spread in the cortical tissue) or has multiple spectral components (e.g. eye movements [119] or sharp transient evoked activity; Figure Ia). Furthermore, it has been argued that the sustained high frequency activity (Figure Ia, upper white box) and the simultaneous oscillatory reduction in lower frequencies (Figure Ia, lower white box) actually reflect the same underlying process: the rotation of the power spectrum ([98,120]; Figure Ib). Likewise, CFC analyses are also hampered by several methodological limitations [40]. For instance, non-sinusoidal brain activity can lead to spurious coupling effects, which might be obscured by band-pass filtering in narrow frequency bands, which will yield an artifactual sinusoidal signal, even if there is no true sinusoidal oscillatory activity present (Figure Ic, [121]).
Taken together, the analysis of oscillatory phase requires a careful inspection of the underlying data. Furthermore, amplitude correlations [57,60] might exhibit similar characteristics and could be applied to both connectivity and CFC analyses [78] and could serve as a useful control analysis [60]. The mutual information framework provides a promising approach to capture non-linear dependencies in electrophysiological data2 ([122], Figure Id), which cannot be described by linear correlation analyses.
Figure I. From linear to non-linear analysis techniques.

(a) Time-frequency analysis of evoked phaselocked (black box) and non-phaselocked (white boxes) activity. (b) Rotation of the power spectrum (black) around a frequency point at approximately 40 Hz might be mistaken as spectral changes in multiple frequency bands (red). Electrophysiological recordings exhibit a prominent 1/f slope (dashed line), which might obscure true oscillatory activity, which is visible as a bump (α/β). (c) The effects of band-pass filtering on non-sinusoidal oscillations: The sensorimotor mu rhythm is rendered sinusoidal by narrow-banded filtering. (d) Exemplary non-linear inverted u-shaped relationship between connectivity and behavior. The graph in a appeared under the Creative Commons Attribution (CC BY) license [28].
Figure 3. Multiplexed cognition: Entrainment and the spatiotemporal organization of goal-directed behavior.
(A) Schematic illustration of how different connectivity metrics might be related. Two hypothetical populations (I and II) could be phase synchronous and exhibit local cross-frequency coupling. Hence, also the amplitudes of the high frequency activity should be correlated over time, which might be reflected in inter-areal PAC (red arrow). However, it is currently unclear whether these phenomena always interact or whether they could occur in isolation. (B) Local CFC: The strength of PAC in frontal and parietal regions correlated with reaction times if attention was deployed to the contralateral hemifield. Circled electrodes show significant effects, the yellow-circled electrode indicates the example electrode. (C) Upper: Directional PAC between the frontal theta-phase and high-gamma in M1. Lower: Directionality was most pronounced at encoding onset and scaled with task-demand. (D) Upper: Directional PAC from PFC to posterior parietal cortex. Lower: Inter-areal theta-gamma PAC was stronger for remembered than forgotten items between frontal seed regions and parieto-occipital EEG sensors. The graph in b appeared under the Creative Commons Attribution (CC BY) license [28]. The graphs in c-d are reproduced with permission from [82,83].
Entrainment as a mechanism of top-down control
The directionality of complex neuronal interactions across several spatiotemporal scales is often difficult to infer, since oscillatory signals are periodic in nature and often lack a defined beginning and may also be confounded by evoked activity. Several methods have been proposed to estimate directionality in electrophysiological recordings (Box 2). Currently, Granger causality (GC, [79]) is amongst one of the most popular techniques. A recent study investigating the PFC-striatum interactions during category learning demonstrated enhanced non-directional beta-band synchrony during category learning (Figure 2B, [54]). In a second step, the authors employed GC to demonstrate that the striatum entrained the PFC and not vice versa.
Box 2. Assessing directionality in connectivity analyses.
It has been suggested that conscious experiences and behavior arises from synchronous activity in widespread (sub-) cortical regions and a variety of measures have been introduced to assess inter-regional neuronal communication [123]. In particular, the CTC hypothesis suggested that areas that exchange information transiently synchronize their activity in distinct narrow frequency bands [4]. However, the information flow through the cortical hierarchy requires that the information flow is directional along defined anatomical pathways. Latency analyses of evoked activity constitute the most commonly accessible approach to trace the flow of neuronal activity through different cortical regions ([21], Figure IIa).
In order to estimate the directionality of the interactions, several methods have been suggested (Figure IIb). Much of the early work employed cross-correlation analyses and generally assumed that the interaction was from A to B, when the lag was smaller than the lag from B to A. Recently, more sophisticated statistical model-based techniques have been suggested, such as Granger causality (GC, [79]) or the phase slope index (PSI, [124]). While GC utilizes an auto-regressive model to predict the future time course, the PSI considers circular dependencies across several temporal scales to infer directionality. Furthermore, directionality can be assessed with the mutual information framework by means of the transfer entropy [123]. In addition, these methods can be used to unravel the directionality of cross-frequency interactions [77,78]. For example, it has recently been demonstrated that the gamma envelope might drive alpha oscillations in parieto-occipital cortex and not vice versa as previously assumed (Figure IIb, [77]).
However, all these metrics are only correlative in nature. Novel brain stimulation tools such as rTMS [5], tACS [18] or direct cortical stimulation [125] allow entraining distinct spectral signatures to subsequently study cross-frequency interactions. Using this approach, it has been demonstrated that both alpha and gamma oscillations might drive CFC interactions, which allows for true bidirectional information integration across temporal scales [78]. In addition, several groups have started to assess inter-regional CFC, where typically the low frequency phase in one area drives high frequency activity in a second area [33,82,83]. Here, directionality is assumed if the directional CFC from A to B is significantly higher than the CFC from B to A.
Figure II. Directional connectivity analyses.

(a) Directionality can be assessed by analyzing onset or peak latencies in different nodes of a network. (b) Oscillatory signals, which are circular in nature, lack a defined beginning and end. Hence, several methods such as cross-correlation, granger causality of the phase slope index have been introduced to infer directionality.
However, how synchrony is established on the network level remains unknown. It has been demonstrated that endogenous burst firing synchronizes PFC and cingulate cortex in lower frequencies during the allocation of attention [80]. In addition, there is emerging evidence that the thalamus plays a key role in establishing widely distributed cortical networks [81]. Given the linkage between high frequency activity (70-200 Hz) and population-spiking activity [58], several studies have addressed directionality by means of directional CFC [33,82]. In contrast to within-region CFC, directional CFC explores the relationship between the oscillatory activity in one region (typically < 20 Hz) and the high frequency activity in another. Converging evidence suggests that prefrontal low frequency oscillations (< 20 Hz) play a key role in organizing large-scale neuronal networks through directed entrainment. In a recent study, it has been demonstrated that PFC-M1 theta-gamma interactions increased with task demand (Figure 3C, [82]). Crucially, they revealed that the frontal theta phase modulated M1 activity, but not vice versa. In addition, they showed that the strength of the directional PAC scaled with task demands and more abstract tasks lead to more PFC-M1 coupling. In contrast to previous studies, they time-locked their directional PAC analyses to the putative encoding onset in PFC. The encoding onset was defined as the time point where the PFC-M1 theta phase relationship exhibited a systematic bias between electrodes, which could have been established through phase resetting. A similar approach was used in a recent study that investigated PFC-cingulate interactions during an attention task [33]. The authors reported that theta in the cingulate cortex entrained high frequency activity in the PFC. Crucially, inter-areal PAC indexed correct attention shifts and might have been dynamically established through phase resetting after burst firing [80]. Theta-gamma directional PAC has also been observed in the fronto-parietal (Figure 3D, [83,84]) and the fronto-thalamic [49] network during memory formation and recall. Taken together, these lines of research suggest that directional PAC potentially reflects a key feature of information transfer and integration across several spatiotemporal scales. Therefore, rhythmic endogenous entrainment might organize the spatiotemporal network dynamics to prioritize neuronal processing in nearby and distant cortical sites.
Linking structural and functional connectivity
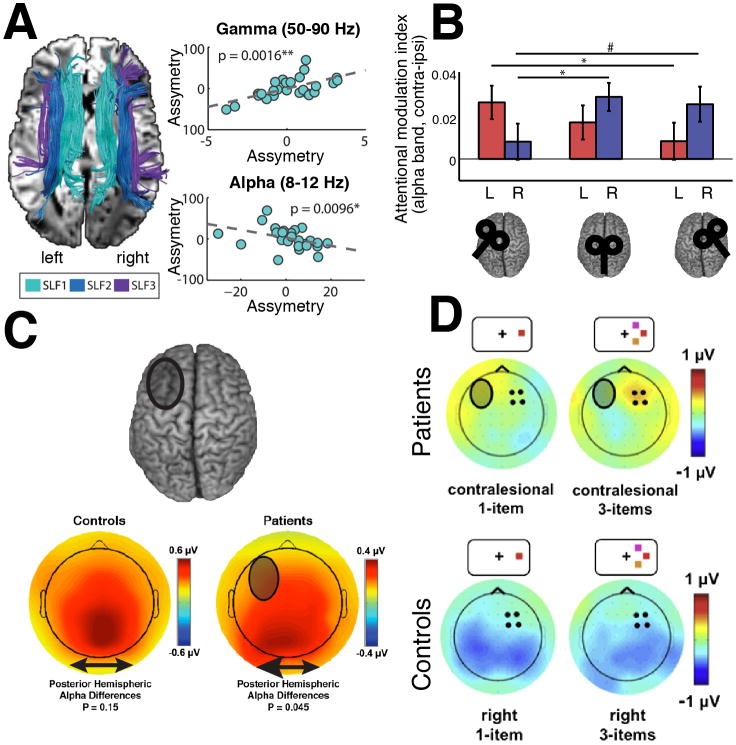
Structural and functional connectivity and their relation to behavior are often studied in isolation. While structural connectivity is mainly assessed by diffusion imaging, functional connectivity can be inferred by circular or linear correlation analyses of band-limited electrophysiological signals (Figure 3A, [3]). Several studies have begun to unravel the structural and functional architecture of the fronto-parietal network and its role for spatial attention [85–87]. Decreased alpha-and increased gamma-synchronization contralateral to the attended hemi field have been suggested to constitute a hallmark of visuospatial attention. Here we review multimodal evidence for the causal relationship of how coupled alpha and gamma oscillations support attentional allocation in the fronto-parietal network. We focus on this well-established network, since several converging studies provide a clear view into how the network is organized [88]. In a first step, they demonstrated that the individual ability to lateralize alpha- and gamma-band synchronization depended on the volume of superior longitudinal fasciculus, which links the PFC with the posterior parietal cortex (PPC; Figure 4A, [86]). The authors then used TMS to causally probe the role of the frontal eye fields (FEF) in PFC for top-down control of oscillatory synchronization in the PPC (Figure 4B, [87]). They convincingly showed that inhibition of the FEF impairs the lateralization of alpha and gamma synchronization. Taken together, both studies suggest that top-down signals from the FEF are mediated via cortico-cortical fibers. Similar findings have been described for inter-hemispheric connectivity in extra-striate areas. Specifically, it has been shown that the integration of a bistable motion stimulus across both visual hemi fields was mediated by callosal fibers connecting bilateral motion-sensitive regions [89]. Subsequently, it has been demonstrated that the instantaneous percept depended on the level of inter-hemispheric gamma-band synchrony, which could selectively be modulated by tACS [90]. Taken together, these findings highlight that synchronized oscillatory brain activity is mediated by cortico-cortical connections between specialized regions to facilitate cortical information transfer and integration within narrow frequency-bands [91–93].
Figure 4. Linking structural and functional connectivity.
(A) Left: Visualization of the superior longitudinal fasciculi (SLF1-3). Right: Asymmetries in white matter volume correlated with the individual ability to lateralize alpha and gamma power in a spatial attention task. (B) Theta-burst TMS to the left FEF, the vertex and the right FEF. The transient TMS induced deactivation of left or right FEF resulted in decreased attentional alpha modulation in the contralateral visual field as compared to the ipsilateral hemifield. (C) Lesions to the prefrontal cortex (grey circle over the left PFC) lead to alpha asymmetries of parieto-occipital EEG sensors with higher alpha power at ipsilateral sensors. (D) Upper: If the lesion hemisphere is challenged (e.g. in a 3 item WM task, upper right) then a compensatory increase in theta power is observed over the non-lesioned PFC, which correlates with electrophysiological signatures over contralateral visual areas. Lower: This effect was restricted to patients with lateralized prefrontal lesions and was not observed in age-matched healthy control group, independent of WM load (1 or 3 items). The graph in a appeared under the Creative Commons Attribution (CC BY) license [86]. The graphs in b-d are reproduced with permission from [87,100,101].
Oscillopathies and network disorders
The synchronization of neuronal oscillations across several spatiotemporal scales constitutes a hallmark of the physiologic brain function [3,7,18]. Hence, numerous neuropsychiatric diseases have been associated with pathological changes of oscillatory processes. In particular, the symptoms of Parkinson's disease (PD) might be caused by abnormal oscillatory activity. For example, Parkinson rigidity has been linked to elevated CFC between the basal ganglia and motor cortex [94]. However, it is currently unclear whether this effect might constitute an artifact of the non-sinusoidal characteristics of beta rhythms in PD [95]. Furthermore, another recent report demonstrated that low frequency tACS that was frequency- and phase-matched to the tremor frequency could reduce the shaking through phase cancellation [96]. Similar oscillatory alterations have been proposed to underlie schizophrenia, autism or attention deficit hyperactivity disorder [97–99].
Here, we consider a simplified model of network disorders, namely circumscribed lesions to key PFC regions. In line with previous findings (Figure 4A/B), a recent lesion study also demonstrated pronounced parieto-occipital alpha asymmetries following unilateral prefrontal lesions (Figure 4C, [100]). Again, this finding highlighted that parieto-occipital alpha oscillations might be under prefrontal top-down control. However, it remained unclear what signal might reflect this top-down influence. A related study described increases in low frequency power in the non-lesioned PFC, but only when the lesioned hemisphere was challenged (Figure 4D, [101]). This finding was interpreted as a dynamic compensatory mechanism and implied that prefrontal activity in the delta/theta range might control parieto-occipital activity. In addition, a monkey lesion study reported that unilateral PFC lesions impaired attentional processes in parieto-occipital cortex, but did not eliminate them [102]. Again, this could indicate a compensation through the intact PFC. This idea had recently been substantiated by the finding that alpha oscillations are co-modulated by a delta rhythm [103], which might arise from the PFC when top-down control was deployed [28,101].
Taken together, the lesion approach allows researchers to causally link spectral signatures to distinct cortical areas and cognitive processes. In the future, this might enable tailored interventions by means of frequency-specific non-invasive brain stimulation to dynamically compensate impaired nodes of the network [18,19].
Concluding Remarks
Neuronal oscillations have been considered an epiphenomenon in the past. However, over the last decade several findings have demonstrated that oscillations guide cortical spiking activity [6] and play a causal role for conscious perception and cognitive processing [5,18,19,104–106]. In particular, several lines of research have provided evidence that cognition emerges from coordinated neuronal activity in specialized yet widely distributed cortical regions [3,4,81]. Although lesion studies have implicated the PFC in cognitive control [1], it remains unclear how the PFC represents goals and provides bias signals to other brain structures [2].
Here we reviewed recent evidence that supports the notion that the PFC employs oscillatory dynamics to coordinate large-scale neuronal interactions, which support the integration of task-relevant goals and rules in activity-silent cortical states [30,107,108] and predict behavior [28,33,38,41,82]. In particular, these findings suggest that phase resetting [33,35] and neuronal entrainment [72,109] reflect key mechanisms of PFC mediated top-down control. Hence, oscillatory dynamics support the multiplexing of different tasks on distinct temporal channels and facilitate the organization of task-relevant coherent networks [69,70], by providing the temporal structure, which may support phase coding by CFC [38,39]. CFC has been proposed to coordinate spatiotemporal dynamics and has been shown to predict behavior on the single trial level [28,82].
Taken together, neuronal oscillations support flexible cognitive processing by recruiting mixed-selective neuronal assemblies into frequency-specific circuits [2,30,64–68,110,111], which then bias distant cortical sites through directed endogenous entrainment [3,7,33,82]. Hence, temporal multiplexing might be ideally suited to subserve cognitive flexibility [65,69]. In particular, the most recent findings highlight the role of slow oscillatory activity for sensory selection, information integration and goal-directed behavior, which might determine the timescale and capacity of cognitive processing [112–115]. In conclusion, accumulating evidence supports the notion that endogenous oscillatory activity in large-scale networks has a causal function for goal-directed behavior and constitutes a promising direction for future research to unravel to core mechanisms of goal-directed behavior (See Outstanding Questions box).
Outstanding Questions Box.
- What is the time scale of cognitive processing? Does the frequency and timing of slow frequency oscillations determine cognitive capacity limitations?
- Do different frequency bands resemble distinct canonical cortical computations? For example, do parieto-occipital alpha oscillations subserve the same purpose as frontal alpha signatures? Do theta oscillations always support memory processes? Are low frequency oscillations always coupled to high frequency activity or could they occur in isolation?
- How do spectral signatures generalize across species? Do higher cognitive functions in humans rely on the same physiologic principles as in non-human primates?
- Most of the cortical processing is rhythmic. However, it is unclear how discrete sampling and periodic processing supports our continuous perception of the world.
- What is the role of non-sinusoidal rhythms for neuronal processing? Does the phase of non-sinusoidal rhythms carry meaningful information? What is the role of the absolute voltage gradient? How does the shape of the power spectrum influence neuronal processing?
- Do different coupling modes (phase- or amplitude-based) reflect distinct cortical entities and how do they relate to each other?
- What is the role of subcortical structures in modulating cortical circuits? In particular, how higher cognitive processes rely on thalamic and striatal regions?
- How do neuronal rhythms relate to spike-timing dependent plasticity? Does endogenous entrainment promote plasticity and do entrained circuits strengthen their synaptic connections? Are synaptic changes frequency-dependent?
- Are neuropsychiatric disorders, such as schizophrenia or ADHD, a result of too little or too much functional connectivity? Can non-invasive brain stimulation be used to therapeutically adjust these connectivity patterns? Are high levels of functional connectivity associated with increased white matter volume?
Trends Box.
- Prefrontal oscillatory dynamics coordinate cortical and subcortical large-scale networks providing a functional basis for flexible cognitive control of goal-directed behavior and do not constitute an epiphenomenon of spiking activity.
- Non-linear dynamics, including phase resetting, endogenous entrainment and cross-frequency coupling, support the spatiotemporal organization of functional networks and predict behavior on the single trial level.
- Neuronal oscillations provide the temporal reference frame for activity-silent encoding in neuronal assemblies, which complements the view that the neuron is the structural and functional unit of the nervous system.
- Multiplexing on different temporal channels reflects distinct canonical computations and increases cortical coding capacity.
- Directionality analyses reveal the timing of information flow along established anatomical pathways.
Acknowledgments
This work was supported by a NINDS Javits Award R37NS21135 (R.T.K.), the Nielsen Corporation (R.T.K.), the German Research Foundation (DFG SFB936/A3/Z1), an intramural research grant from the Dept. of Psychology, University of Oslo and the Alexander von Humboldt Foundation (Feodor Lynen Program, R.F.H.).
Glossary
- Activity-silent processing
Traditionally, active processing has been associated with an increase in neuronal spiking or high frequency activity. However, meaningful processing with behavioral consequences has also been observed without changes in spiking and are referred to as activity-silent.
- Cognitive control
The term summarized various executive functions, such as attention, working memory, error monitoring, inhibitory control or planning, which reflect the means to achieve fluid behavior.
- Cross-frequency coupling (CFC)
CFC describes a systemic correlation between two oscillations with different frequencies. The majority of CFC is assesses by phase-amplitude-coupling (PAC), where the phase of slow oscillations correlates with the amplitude of a faster oscillation.
- Functional connectivity (FC)
A measure of interaction between two signals based on their amplitude or phase relationships. Most commonly, FC is assessed by coherence or phase-locking analyses of band-limited signals or by linear correlations of the amplitude/power time series.
- High frequency activity (HFA)
Also referred to as high gamma, describes activity in the 70-200 Hz range that is commonly observed in the LFP (local field potential) of ECoG (electrocorticography) studies and closely correlates with population spiking activity. It is often used to infer whether a cortical region is actively engaged in a task or not.
- Entrainment
Describes the directed synchronization of one oscillator by another. Exogenous entrainment occurs when brain oscillations adapt their rhythm to track an exogenous periodicity. Endogenous entrainment explains how one region might drive activity in a second region.
- Non-invasive brain stimulation (NIBS)
Here, we mainly refer to rTMS (rhythmic transcranial magnetic stimulation) and tACS (transcranial alternating current stimulation), which both have been suggested to entrain frequency-specific activity to causally link neuronal oscillations to distinct cognitive processes.
- Phase resetting
Refers to a process where the phase of the ongoing band-limited brain activity is adjusted to a certain angle by either an external or internal cue.
- Phase coding
Constitutes an elegant mechanism to increase cortical coding capacity. Activity by the same neuronal population might reflect distinct pieces of information, depending on when the activity occurs relative to the phase of the band-limited local field potential (LFP)
Footnotes
VanRullen, R. (2016) How to evaluate phase differences between trial groups in ongoing electrophysiological signals. bioRxiv DOI: 10.1101/061283
Ince, R.A.A. et al. (2016) A statistical framework for neuroimaging data analysis based on mutual information estimated via a Gaussian copula. bioRxiv DOI: 10.1101/043745
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83:1002–1018. doi: 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 3.Siegel M, et al. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- 4.Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thut G, et al. The functional importance of rhythmic activity in the brain. Curr Biol CB. 2012;22:R658–663. doi: 10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 8.Engel AK, et al. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 9.Varela F, et al. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 10.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaak E, et al. Local entrainment of α oscillations by visual stimuli causes cyclic modulation of perception. J Neurosci Off J Soc Neurosci. 2014;34:3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiebelkorn IC, et al. Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr Biol CB. 2013;23:2553–2558. doi: 10.1016/j.cub.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau AN, Fries P. Attention samples stimuli rhythmically. Curr Biol CB. 2012;22:1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Song K, et al. Behavioral oscillations in attention: rhythmic α pulses mediated through θ band. J Neurosci Off J Soc Neurosci. 2014;34:4837–4844. doi: 10.1523/JNEUROSCI.4856-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyart V, et al. Rhythmic fluctuations in evidence accumulation during decision making in the human brain. Neuron. 2012;76:847–858. doi: 10.1016/j.neuron.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leszczyński M, et al. Rhythmic Working Memory Activation in the Human Hippocampus. Cell Rep. 2015;13:1272–1282. doi: 10.1016/j.celrep.2015.09.081. [DOI] [PubMed] [Google Scholar]
- 18.Fröhlich F, et al. Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert Rev Neurother. 2015;15:145–167. doi: 10.1586/14737175.2015.992782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann CS, et al. EEG oscillations: From correlation to causality. Int J Psychophysiol Off J Int Organ Psychophysiol. 2016;103:12–21. doi: 10.1016/j.ijpsycho.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 20.VanRullen R. Perceptual Cycles. Trends Cogn Sci. 2016 doi: 10.1016/j.tics.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Siegel M, et al. Cortical information flow during flexible sensorimotor decisions. Science. 2015;348:1352–1355. doi: 10.1126/science.aab0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisz N, et al. Prestimulus oscillatory power and connectivity patterns predispose conscious somatosensory perception. Proc Natl Acad Sci U S A. 2014;111:E417–425. doi: 10.1073/pnas.1317267111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes M, Spaak E. The Importance of Single-Trial Analyses in Cognitive Neuroscience. Trends Cogn Sci. 2016;20:483–486. doi: 10.1016/j.tics.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Latimer KW, et al. NEURONAL MODELING. Single-trial spike trains in parietal cortex reveal discrete steps during decision-making. Science. 2015;349:184–187. doi: 10.1126/science.aaa4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundqvist M, et al. Gamma and Beta Bursts Underlie Working Memory. Neuron. 2016;90:152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16:487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 27.Katz LN, et al. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature. 2016;535:285–288. doi: 10.1038/nature18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szczepanski SM, et al. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biol. 2014;12:e1001936. doi: 10.1371/journal.pbio.1001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen MX. Fluctuations in oscillation frequency control spike timing and coordinate neural networks. J Neurosci Off J Soc Neurosci. 2014;34:8988–8998. doi: 10.1523/JNEUROSCI.0261-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokes MG. “Activity-silent” working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19:394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dürschmid S, et al. Hierarchy of prediction errors for auditory events in human temporal and frontal cortex. Proc Natl Acad Sci U S A. 2016;113:6755–6760. doi: 10.1073/pnas.1525030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonken YM, et al. Frontal and motor cortex contributions to response inhibition: evidence from electrocorticography. J Neurophysiol. 2016;115:2224–2236. doi: 10.1152/jn.00708.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voloh B, et al. Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts. Proc Natl Acad Sci U S A. 2015;112:8457–8462. doi: 10.1073/pnas.1500438112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daitch AL, et al. Frequency-specific mechanism links human brain networks for spatial attention. Proc Natl Acad Sci U S A. 2013;110:19585–19590. doi: 10.1073/pnas.1307947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney-Reed CM, et al. Thalamic theta phase alignment predicts human memory formation and anterior thalamic cross-frequency coupling. eLife. 2015;4 doi: 10.7554/eLife.07578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen MX, Donner TH. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J Neurophysiol. 2013;110:2752–2763. doi: 10.1152/jn.00479.2013. [DOI] [PubMed] [Google Scholar]
- 37.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watrous AJ, et al. Phase-amplitude coupling supports phase coding in human ECoG. eLife. 2015;4 doi: 10.7554/eLife.07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel M, et al. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci U S A. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aru J, et al. Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol. 2015;31:51–61. doi: 10.1016/j.conb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Buschman TJ, et al. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nácher V, et al. Coherent delta-band oscillations between cortical areas correlate with decision making. Proc Natl Acad Sci U S A. 2013;110:15085–15090. doi: 10.1073/pnas.1314681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacchet MD, et al. Attention drives synchronization of alpha and beta rhythms between right inferior frontal and primary sensory neocortex. J Neurosci Off J Soc Neurosci. 2015;35:2074–2082. doi: 10.1523/JNEUROSCI.1292-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liebe S, et al. Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci. 2012;15:456–462. S1–2. doi: 10.1038/nn.3038. [DOI] [PubMed] [Google Scholar]
- 45.Gregoriou GG, et al. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips JM, et al. A long-range fronto-parietal 5- to 10-Hz network predicts “top-down” controlled guidance in a task-switch paradigm. Cereb Cortex N Y N. 2014;199124:1996–2008. doi: 10.1093/cercor/bht050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldauf D, Desimone R. Neural mechanisms of object-based attention. Science. 2014;344:424–427. doi: 10.1126/science.1247003. [DOI] [PubMed] [Google Scholar]
- 48.Micheli C, et al. Inferior-frontal cortex phase synchronizes with the temporal-parietal junction prior to successful change detection. NeuroImage. 2015;119:417–431. doi: 10.1016/j.neuroimage.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney-Reed CM, et al. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. eLife. 2014;3:e05352. doi: 10.7554/eLife.05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson KL, et al. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex N Y N. 2010;199120:1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- 51.Backus AR, et al. Hippocampal-Prefrontal Theta Oscillations Support Memory Integration. Curr Biol CB. 2016;26:450–457. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- 52.Crespo-García M, et al. Slow-theta power decreases during item-place encoding predict spatial accuracy of subsequent context recall. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Hanslmayr S, et al. Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr Biol CB. 2014;24:904–909. doi: 10.1016/j.cub.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83:216–225. doi: 10.1016/j.neuron.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brincat SL, Miller EK. Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci. 2015;18:576–581. doi: 10.1038/nn.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watrous AJ, et al. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci. 2013;16:349–356. doi: 10.1038/nn.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hipp JF, et al. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15:884–890. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray S, Maunsell JHR. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermes D, et al. Stimulus Dependence of Gamma Oscillations in Human Visual Cortex. Cereb Cortex N Y N. 2015;199125:2951–2959. doi: 10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Nicolai C, et al. Corticostriatal coordination through coherent phase-amplitude coupling. J Neurosci Off J Soc Neurosci. 2014;34:5938–5948. doi: 10.1523/JNEUROSCI.5007-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helfrich RF, et al. Spectral fingerprints of large-scale cortical dynamics during ambiguous motion perception. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vosskuhl J, et al. Increase in short-term memory capacity induced by down-regulating individual theta frequency via transcranial alternating current stimulation. Front Hum Neurosci. 2015;9:257. doi: 10.3389/fnhum.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polanía R, et al. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol CB. 2012;22:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 64.Rigotti M, et al. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fusi S, et al. Why neurons mix: high dimensionality for higher cognition. Curr Opin Neurobiol. 2016;37:66–74. doi: 10.1016/j.conb.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Stokes MG, et al. Dynamic coding for cognitive control in prefrontal cortex. Neuron. 2013;78:364–375. doi: 10.1016/j.neuron.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller EK, Fusi S. Limber neurons for a nimble mind. Neuron. 2013;78:211–213. doi: 10.1016/j.neuron.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blackman RK, et al. Monkey Prefrontal Neurons Reflect Logical Operations for Cognitive Control in a Variant of the AX Continuous Performance Task (AX-CPT) J Neurosci Off J Soc Neurosci. 2016;36:4067–4079. doi: 10.1523/JNEUROSCI.3578-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akam T, Kullmann DM. Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat Rev Neurosci. 2014;15:111–122. doi: 10.1038/nrn3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knight RT, Eichenbaum H. Multiplexed memories: a view from human cortex. Nat Neurosci. 2013;16:257–258. doi: 10.1038/nn.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Place R, et al. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci. 2016 doi: 10.1038/nn.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bastos AM, et al. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 73.Michalareas G, et al. Alpha-Beta and Gamma Rhythms Subserve Feedback and Feedforward Influences among Human Visual Cortical Areas. Neuron. 2016 doi: 10.1016/j.neuron.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmiedt JT, et al. Beta oscillation dynamics in extrastriate cortex after removal of primary visual cortex. J Neurosci Off J Soc Neurosci. 2014;34:11857–11864. doi: 10.1523/JNEUROSCI.0509-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hillebrand A, et al. Direction of information flow in large-scale resting-state networks is frequency-dependent. Proc Natl Acad Sci U S A. 2016;113:3867–3872. doi: 10.1073/pnas.1515657113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alekseichuk I, et al. Spatial Working Memory in Humans Depends on Theta and High Gamma Synchronization in the Prefrontal Cortex. Curr Biol CB. 2016;26:1513–1521. doi: 10.1016/j.cub.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 77.Jiang H, et al. Measuring directionality between neuronal oscillations of different frequencies. NeuroImage. 2015;118:359–367. doi: 10.1016/j.neuroimage.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 78.Helfrich RF, et al. Different coupling modes mediate cortical cross-frequency interactions. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 79.Ding M, et al. Granger Causality: Basic Theory and Application to Neuroscience. In: Schelter B, et al., editors. Handbook of Time Series Analysis. Wiley-VCH Verlag GmbH & Co. KGaA; 2006. pp. 437–460. [Google Scholar]
- 80.Womelsdorf T, et al. Burst firing synchronizes prefrontal and anterior cingulate cortex during attentional control. Curr Biol CB. 2014;24:2613–2621. doi: 10.1016/j.cub.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 81.Saalmann YB, et al. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voytek B, et al. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat Neurosci. 2015;18:1318–1324. doi: 10.1038/nn.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friese U, et al. Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. NeuroImage. 2013;66:642–647. doi: 10.1016/j.neuroimage.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Köster M, et al. Theta-gamma coupling during episodic retrieval in the human EEG. Brain Res. 2014;1577:57–68. doi: 10.1016/j.brainres.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 85.Jensen O, et al. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014;37:357–369. doi: 10.1016/j.tins.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Marshall TR, et al. Frontoparietal Structural Connectivity Mediates the Top-Down Control of Neuronal Synchronization Associated with Selective Attention. PLoS Biol. 2015;13:e1002272. doi: 10.1371/journal.pbio.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marshall TR, et al. Frontal eye fields control attentional modulation of alpha and gamma oscillations in contralateral occipitoparietal cortex. J Neurosci Off J Soc Neurosci. 2015;35:1638–1647. doi: 10.1523/JNEUROSCI.3116-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szczepanski SM, et al. Functional and structural architecture of the human dorsal frontoparietal attention network. Proc Natl Acad Sci U S A. 2013;110:15806–15811. doi: 10.1073/pnas.1313903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Genç E, et al. Interhemispheric connections shape subjective experience of bistable motion. Curr Biol CB. 2011;21:1494–1499. doi: 10.1016/j.cub.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Helfrich RF, et al. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol. 2014;12:e1002031. doi: 10.1371/journal.pbio.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chanes L, et al. Causal frequency-specific contributions of frontal spatiotemporal patterns induced by non-invasive neurostimulation to human visual performance. J Neurosci Off J Soc Neurosci. 2013;33:5000–5005. doi: 10.1523/JNEUROSCI.4401-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quentin R, et al. Fronto-Parietal Anatomical Connections Influence the Modulation of Conscious Visual Perception by High-Beta Frontal Oscillatory Activity. Cereb Cortex N Y N. 2015;199125:2095–2101. doi: 10.1093/cercor/bhu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quentin R, et al. Visual Contrast Sensitivity Improvement by Right Frontal High-Beta Activity Is Mediated by Contrast Gain Mechanisms and Influenced by Fronto-Parietal White Matter Microstructure. Cereb Cortex N Y N. 2016;199126:2381–2390. doi: 10.1093/cercor/bhv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swann NC, et al. Elevated synchrony in Parkinson disease detected with electroencephalography. Ann Neurol. 2015;78:742–750. doi: 10.1002/ana.24507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lozano-Soldevilla D, et al. Neuronal Oscillations with Non-sinusoidal Morphology Produce Spurious Phase-to-Amplitude Coupling and Directionality. Front Comput Neurosci. 2016;10:87. doi: 10.3389/fncom.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brittain JS, et al. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol CB. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry. 2015;77:1001–1009. doi: 10.1016/j.biopsych.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 98.Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry. 2015;77:1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calderone DJ, et al. Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn Sci. 2014;18:300–309. doi: 10.1016/j.tics.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci U S A. 2010;107:18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voytek B, et al. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gregoriou GG, et al. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat Neurosci. 2014;17:1003–1011. doi: 10.1038/nn.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wöstmann M, et al. Spatiotemporal dynamics of auditory attention synchronize with speech. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1523357113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thut G, et al. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol. 2011;2:170. doi: 10.3389/fpsyg.2011.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klimesch W, et al. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17:1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- 106.Helfrich RF, et al. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol CB. 2014;24:333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 107.Myers NE, et al. Oscillatory brain state predicts variability in working memory. J Neurosci Off J Soc Neurosci. 2014;34:7735–7743. doi: 10.1523/JNEUROSCI.4741-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wimmer K, et al. Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits. J Neurosci Off J Soc Neurosci. 2016;36:489–505. doi: 10.1523/JNEUROSCI.3678-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bosman CA, et al. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rutishauser U, et al. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 111.Koechlin E, et al. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 112.Libet B. The timing of mental events: Libet's experimental findings and their implications. Conscious Cogn. 2002;11:291–299. 333. doi: 10.1006/ccog.2002.0568. [DOI] [PubMed] [Google Scholar]
- 113.Lisman JE, Idiart MA. Storage of 7 +/- 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 114.Lisman JE, Jensen O. The θ-γ neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buschman TJ, et al. Neural substrates of cognitive capacity limitations. Proc Natl Acad Sci U S A. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berens P. CircStat: A MATLAB Toolbox for Circular Statistics. J Stat Softw. 2009;31:21. [Google Scholar]
- 117.van der Meij R, et al. Phase-amplitude coupling in human electrocorticography is spatially distributed and phase diverse. J Neurosci Off J Soc Neurosci. 2012;32:111–123. doi: 10.1523/JNEUROSCI.4816-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lowet E, et al. Areas V1 and V2 show microsaccade-related 3-4-Hz covariation in gamma power and frequency. Eur J Neurosci. 2016;43:1286–1296. doi: 10.1111/ejn.13126. [DOI] [PubMed] [Google Scholar]
- 120.Freeman WJ, Zhai J. Simulated power spectral density (PSD) of background electrocorticogram (ECoG) Cogn Neurodyn. 2009;3:97–103. doi: 10.1007/s11571-008-9064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fransen AMM, et al. Identifying neuronal oscillations using rhythmicity. NeuroImage. 2015;118:256–267. doi: 10.1016/j.neuroimage.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 122.Schyns PG, et al. Cracking the code of oscillatory activity. PLoS Biol. 2011;9:e1001064. doi: 10.1371/journal.pbio.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bastos AM, Schoffelen JM. A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls. Front Syst Neurosci. 2015;9:175. doi: 10.3389/fnsys.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nolte G, et al. Robustly estimating the flow direction of information in complex physical systems. Phys Rev Lett. 2008;100:234101. doi: 10.1103/PhysRevLett.100.234101. [DOI] [PubMed] [Google Scholar]
- 125.Alagapan S, et al. Modulation of Cortical Oscillations by Low-Frequency Direct Cortical Stimulation Is State-Dependent. PLoS Biol. 2016;14:e1002424. doi: 10.1371/journal.pbio.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]