Abstract
This paper presents unique data on the reproductive behavior of the rare giant armadillo (Priodontes maximus), including gestation, inter-birth intervals, number of offspring and parental care. It also describes a potential non-parental infanticide. The study used telemetry, camera traps and track observations for over 7 years in a 300-km2 area in the central Brazilian Pantanal. Females with young were recorded 5 times. Reproductive events did not appear to be seasonal. A 5-month gestation period was estimated. Parental care is long, as the offspring is completely dependent on its mother’s milk until 6–8 months of age. Weaning was estimated to occur at 11–12 months, but the offspring continued to be dependent on its mother’s burrows until 18 months old. Three births were recorded over a 6-year period for one individual. The offspring from the first birth recorded was killed at 4 weeks of age in a potential infanticide, but 7 months after the first birth, a second offspring was born. A third birth was recorded 3 years after the second birth. Results from this study suggest that the population growth rate of giant armadillos is very low and the species can therefore easily be locally extirpated.
Introduction
The giant armadillo [Priodontes maximus (Kerr, 1792)], the largest of living cingulates (Mammalia: Cingulata), can reach up to 150 cm (head to tail) and weigh up to 50 kg (Emmons and Feer 1997, Eisenberg and Redford 1999, Desbiez et al. 2019). One of the most striking features of the species is the large scimitar-shaped fore-claws, the third of which is greatly enlarged and can be as long as 20.3 cm (Carter et al. 2016). Giant armadillos are found over much of South America – in 11 different countries – in habitats ranging from tropical forest to open savanna (Abba and Superina 2010). Although widespread, giant armadillos are rare (Meritt 2006), and are currently classified as “Vulnerable” (A2cd) on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (Anacleto et al. 2014).
Giant armadillos are nocturnal and fossorial, and rarely seen, even by local people. All studies confirm the rarity of the species (Walsh and Gannon 1967, Noss et al. 2004, Silveira et al. 2009, Srbek-Araujo et al. 2009). Their large burrows are often the only evidence of their presence and the focus of several studies (Carter 1983, Carter and Encarnação 1983, Anacleto 1997, Ceresoli and Fernandez-Duque 2012, Porfirio et al. 2012). The role they play in the ecological community as ecosystem engineers has been described for various biomes (Leite Pitman et al. 2004), (Desbiez and Kluyber 2013, Aya-Cuero et al. 2017, Massocato and Desbiez 2017). Despite being the object of quite a number of publications (Superina et al. 2014), the overall life-history data for giant armadillos are lacking, and reports on the basic reproductive characteristics (Merrett 1983) are probably inaccurate (Aya-Cuero et al. 2015, Carter et al. 2016). Despite a misleading picture in Gijzen (1965), the species has never bred in captivity (ZIMS 2017). The only information on reproduction comes from Aya-Cuero et al. (2015), who, using camera traps, obtained three records of giant armadillo females with single offspring in Colombia.
Conservation strategies for giant armadillos must be based on the characteristics of their ecology and biology that impact their population dynamics the most. Analysis of the population growth rate and its determinants is key to understanding population dynamics, as population growth rate is the key unifying variable that links the various aspects of a species’ population ecology (Sibly and Hone 2002). A basic understanding of a species’ reproductive rate is necessary for the assessment of population growth rates. This paper presents some of the first data on the reproductive behavior of giant armadillos, including reproductive seasonality, gestation period, inter-birth intervals, number of offspring and parental care. It also discusses a possible non-parental infanticide.
Materials and methods
Study area
This study was carried out between July 2010 and July 2017, in a 300-km2 area that includes seven traditionally managed cattle ranches (19° 16′ 60″ S, 55° 42′ 60″ W) in the Nhecolândia sub-region of the Brazilian Pantanal. The landscape is a mosaic of different habitats that include open grassland, scrub grassland, scrub forest and semi-deciduous forest. The historical mean temperature is 25.4°C, climate is classified as semi-humid tropical (Aw), with a hot, rainy season (October to March) and a warm drier season (April to September) during which temperatures may drop due to cold fronts from the South (Soriano 2000). The area lacks permanent watercourses, and there is widespread flooding during the rainy season. Traditional extensive cattle ranching is practiced in the area, and there are no paved roads. Hunting and habitat loss is limited, and the overall anthropogenic threats are low.
Capture and handling
We performed active searches by foot or pickup trucks looking for signs (tracks, feces and burrows) of giant armadillos that could lead to a burrow in use. Animals were captured using iron funnel traps that were placed in the entrance of burrows with evidence of recent activity. We captured 26 giant armadillos in the study area, 14 females (10 adults and four subadults) and 12 males (seven adults, four subadults and one juvenile). Once captured, animals were temporarily placed in ventilated wooden boxes and then anesthetized through an intramuscular injection in the hind limbs. The anesthetic protocol was composed of butorphanol 10 mg/ml (0.1 mg/kg), detomidine 10 mg/ml (0.1 mg/kg) and midazolam 5 mg/ml (0.2 mg/kg) (Kluyber 2016). While immobilized, information on age, sex, any evidences of reproductive activity and any natural marks that could allow visual identification of the individual in the future were collected. Skin biopsies were performed to allow genetic kinship studies in the future.
Armadillos were implanted with intra-abdominal very-high-frequency (VHF) radio transmitters (IMP 310, Telonics, Inc., Mesa, AZ, USA – weight=38.5 g, i.e. 1.28% of armadillo’s body mass) following the surgical procedures proposed by Hernandez et al. (2010). Once all procedures were terminated, anesthesia was reversed through an intravenous injection of naloxone (0.04 mg/kg), yohimbine (0.125 mg/kg) and flumazenil (0.025 mg/kg). Animals were released in the same burrow they were captured from.
Animal monitoring
Animals were monitored for an average of 15 days per month. Monitoring was accomplished through VHF telemetry, to find the burrow using the homing-in to the animal technique (Samuel and Fuller 1994). Once encountered, the burrow location was recorded using a handheld global positioning system (GPS) device. To allow the identification and the comparison between “regular” burrows and “nesting” burrows (i.e. burrows used to shelter infants), the burrow entrance height and width were measured following the protocol used by Desbiez and Kluyber (2013). In addition, the amount of sand in front of a burrow was measured and characterized by the height of the sand mound (from the soil level to the top of the mound). Animals were captured and anesthetized under the license number 27587-8 from the Brazilian Federal Environmental Protection Agency (ICMBIO), which regulates and protects wildlife in the country.
Camera trapping
We used camera traps to record the different types of behavior performed by individuals with and without offspring near burrow entrances and eventual visits of other individuals to those burrows. When burrows in use were encountered, either through telemetry or active searches, camera traps were placed in front of its entrances and set to take a succession of pictures (Reconyx® HC-500, Holmen, WI, USA) or set to video mode (Bushnell® Trophy Camera Brown Model, Overland Park, Kansas, USA and Reconyx® XP9, Holmen, WI, USA) as described in Desbiez and Kluyber (2013). Camera traps remained set in front of burrows for at least 40 days after the monitored individuals left it (5769 camera traps/night). Through this method, we were able to record the time an individual entered and exited the burrow and estimate the time spent inside and outside the burrow in each outing. For individuals with offspring, camera traps allowed us to make observations of parental care, such as closing the burrow’s entrance upon each departure and playing behavior, and changes in the infant’s behavior over time. In an attempt to allow the infant’s age estimation through changes in behavior of the mother and offspring over time, we categorized the recorded behaviors and associated them with the infant’s age at the time they were recorded. This method could only be applied to two reproductive events that could be closely monitored (Supplementary material 1). To evaluate if behaviors such as the time spent inside or outside the burrow and the closing of the burrow’s entrance changed as the offspring grew older, we used linear regressions. To evaluate the non-linear relationship between the time spent outside the burrow and offspring growth, we fitted a cubic polynomial model. Due to the limited number of records, behavioral observations were grouped into 2-week intervals.
Individual identification and morphological changes of the infants through time
The giant armadillo’s carapace extends about halfway down its sides, being dark brown to black dorsally and light colored near the edges (Carter et al. 2016). When recorded in camera traps, individuals of Priodontes maximus were identified through variations in scale coloring pattern, such as the number and arrangement of light and dark scales in the carapace and tail (Noss et al. 2004) and other natural marks, such as scars. As reported by Aya-Cuero et al. (2015), young individuals have comparatively lighter-colored carapace, and here we recorded the changes in carapace coloring of one young individual of known age. The patterns of coloring and behavioral change chronicled were used in an attempt to estimate the age of other young individuals of unknown age observed during our study.
Results
Reproductive events
Reproductive events did not appear to be seasonal and were recorded through camera traps for four out of the 10 adult females monitored (Table 1). For one of these females (F4), reproductive behavior was monitored in detail through intensive telemetry monitoring and camera trapping (Supplementary material 1). This female gave birth 3 times in a 6-year period. In addition, reproductive activity was identified for one other female, which was photographed by camera traps closely accompanied by a young individual.
Reproductive events recorded for five female giant armadillos (Priodontes maximus) between 2010 and 2017 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
| Reproductive event | Id of female (mother) | Approximate parturition date | Sex of offspring | Fate of offspring |
|---|---|---|---|---|
| 1 | F4 | November (2012) | M | D |
| 2 | F4 | July (2013) | M | D |
| 3 | F4 | August (2016) | M | U |
| 4 | F15 | ±February to May (2012) | F (F19) | A |
| 5 | F24 | ±February to August (2016) | M | A |
| 6 | F27 | ±December 2016 | U | U |
| 7 | F – not captured | ±December (2012) to February (2013) | F (F16) | A |
Approximate date of parturition of each female’s offspring is estimated based on its size, behavior and coloration when recorded (see Table 2). We also incorporated information on the offspring’s sex (male; female) and fate (dead; alive; unknown).
Inter-birth interval
The first birth recorded for F4 was at the end of 2012 (Table 1); however, the infant died 4 weeks after birth. A second birth occurred 7 months after the death of the infant, and 8 months after the first recorded parturition event. The second infant (M17) was monitored until it was a 2-year-old juvenile, when it was injured, presumably by a puma (Puma concolor), and died. Finally, 36 months after the second birth, and 13 months after the death of that offspring (M17), F4 gave birth to a third infant, which was monitored until it was 5 months old. Due to severe flooding in the study area, we were unable to continue monitoring F4 and its offspring after December 2016.
Infant survival and causes of death
From the seven reproductive events recorded, two have unknown fates, two infant males died, and two females and one male survived. Survivors continue to be sporadically monitored through camera traps (Table 1). Two infants (one male and one of unknown sex) had a limited monitoring period and we cannot state if they survived or not after their last record by our team. One male died from a predation attempt at 2 years of age (F4’s second offspring), while the other male died at 4 weeks of age (F4’s first offspring), in a potential non-parental infanticide. An adult male (M14) entered the nesting burrow after chasing out the mother (F4) and remained for 42 consecutive hours inside the nesting burrow with the offspring. After this period, this infant was found dead. When M14 chased F4 out of the burrow, he was also recorded trying to climb on top of her in an apparent mating attempt. Through carapace marks and camera trap records, we could identify this male individual and confirm that it is not the same individual (M5) thought to have sired the killed offspring (see Supplementary material 2 for a full description of the recorded behavior).
Pairing behavior and gestation period
From the 26 giant armadillos monitored during a 7-year period, adult individuals were only recorded to share a burrow on one occasion. A non-resident adult male (M5) sporadically visited the home range, and specifically, the old burrows used by the resident adult female (F4). However, for 2 days, in June 2012, M5 and F4 were recorded sharing a burrow in which they were documented entering and leaving together. Five months later, in November 2012, camera traps placed in front of F4’s burrow documented behavioral changes and the birth of its first documented offspring (Supplementary material 1).
Parental care behavior
All monitored giant armadillos in this project generally changed burrows every night or every couple of nights. Female giant armadillos began consistently re-using their burrows only when they had an offspring. The longest periods of burrow reuse recorded were those observed immediately after F4’s first (at least 22 days) and second parturitions (24 days). Throughout most of the first year of an offspring, the female returned periodically to the burrow where its offspring was kept. It nursed its infant inside the burrow and after a period of time, it guided the infant to a new nesting burrow. For example, F4’s second infant (M17) remained in a nesting burrow for 5–20 days (mean=14), before being taken to a new nesting burrow. Only 12 burrows were used during the first 6 months of M17’s life. New nesting burrows were located 20–300 m away from the previous burrow where M17 had been sheltered. During burrow changes, F4 would build a ramp at the burrow entrance and guide M17, often walking backwards and nuzzling it (https://www.youtube.com/watch?v=TolnJ9ISarU). This behavior was recorded for all offspring less than 6 months. Even though video camera traps were equipped with microphones, no sonorous communication between the female and infant were detected.
During the first 10 weeks of M17’s life, when leaving the burrow for its nightly foraging activities, F4 would close the burrow entrance by collecting sand with its front paws and pushing it back with its back paws to close the entrance (Figure 1). F4 was also recorded to use the flattened area on the top of her skull to forcefully seal the nesting burrow’s entrance. This behavior decreased in frequency as the infants got older (R2=0.86; F=64.18; p<0.01; Figure 2). This burrow entrance sealing behavior was never recorded for a giant armadillo without offspring.

Sequence of camera trap photographs illustrating the burrow entrance sealing behavior by female giant armadillos (Priodontes maximus) with young offspring.
When (A) females leave the burrow where the infant is, she (B) digs with her front claws into the sand and kicks it back to seal the entrance, (C) leaving only once the entrance is fully sealed with sand.
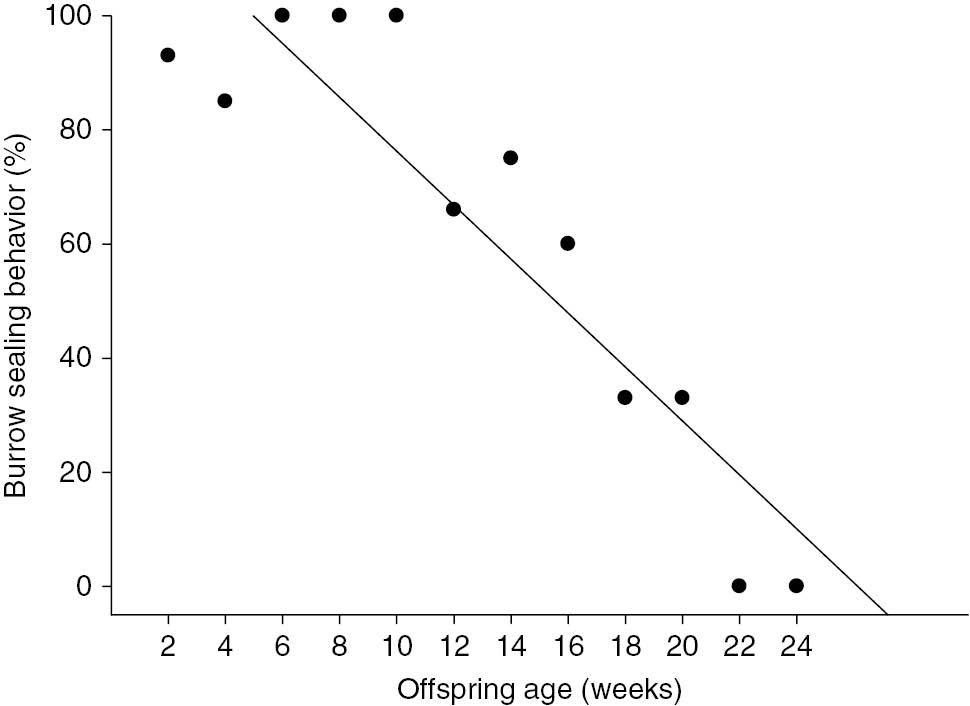
Frequency of burrow entrance sealing behavior upon leaving the burrow according to offspring’s age in weeks.
Data from one adult female giant armadillo Priodontes maximus (F4) monitored between July and December 2013 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
The time spent by F4 inside and outside the nesting burrow was also related to M17’s age (Figures 3 and 4). In the first days after parturition, F4 would remain inside the burrow for 18.5±1.57 h (min=16, max=21 h) and outside it (presumably foraging) for 5.5±1.14 h (min=4, max=8). However, the time spent on each outing was positively correlated with M17’s age and increased gradually and then leveled off at 16 weeks of age (R2=0.88, F=136.6, p<0.01; Figure 3). After the first 2 weeks, F4 spent 18–19 h with M17 and then left for 30 h (resting in a different burrow after foraging activities). When M17 got older, intervals of up to 80 h were recorded between encounters with F4. The length of time F4 spent with M17 inside the nesting burrow also decreased as M17 got older (R2=0.11; F=6.92; p=0.01; Figure 4).
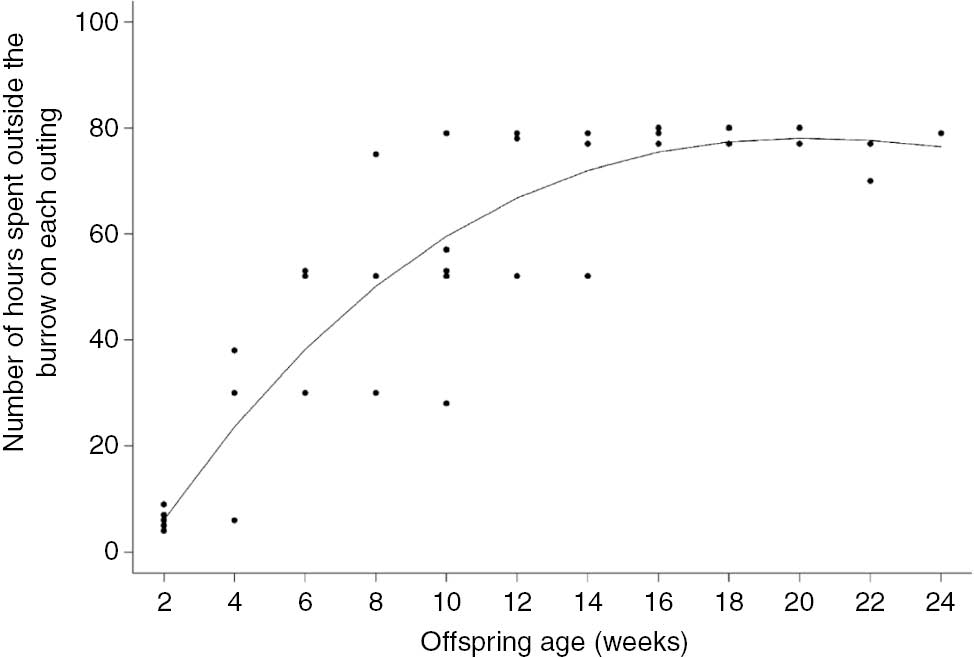
Time (hours) spent by an adult female outside the nesting burrow (i.e. away from its offspring), during each outing, according to its offspring’s age (2-week intervals).
Data from one adult female giant armadillo Priodontes maximus (F4) monitored between July and December 2013 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
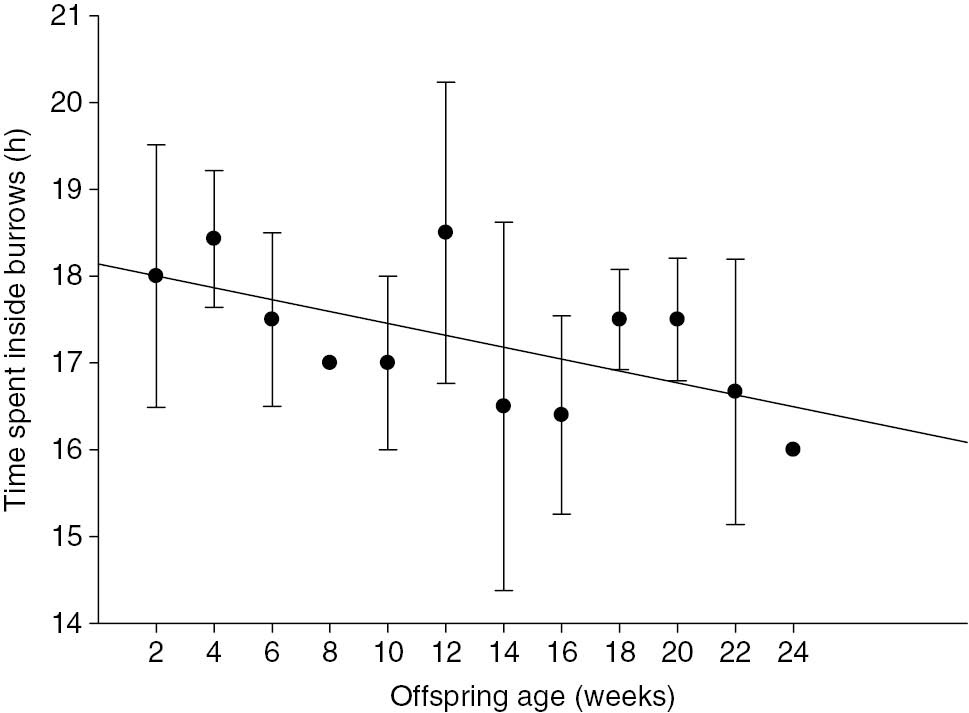
Average time (hours) spent by an adult female inside the nesting burrow (i.e. with its offspring), between its outings, according to its offspring’s age (2-week intervals).
Data from one adult female giant armadillo Priodontes maximus (F4) monitored between July and December 2013 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
The first records of playing behavior between the adult female and infant were recorded on the sand mound in front of a nesting burrow when M17 was 5 months old. The playing behavior consisted of the infant climbing onto its mother (in a similar manner as described by Aya-Cuero et al. 2015), nuzzling between the adult female and infant, and both individuals rolling on their backs and chasing each other.
Morphological changes in offspring
As infants of giant armadillos presented a light-colored carapace and cephalic shield that became gradually darker as they got older (Figure 5), an offspring’s morphological changes can be used to estimate its age (Tables 2 and 3). Infant size could only be estimated for M17 at 24 days of age (the first time it came out of its burrow after birth). It was estimated to have an approximate body length of 40–45 cm, from the tip of the snout to the tip of the tail. The first time M17 was photographed with its eyes open was at 53 days of age. M17 had its eyes closed at 43 days old, so the eyes should have opened between 43 and 53 days of age. Based on these parameters, F4’s first infant was killed at less than 43–53 days of age, as its eyes were still closed.

Changes in the coloration of the infant giant armadillo (Priodontes maximus) over time.
Coloration at: (A) 24 days old; (B) 31 days old; (C) 43 days old (notice eyes are closed) (D) 53 days old (notice the eyes are open); (E) 78 days old; (F) 106 days old. Records of two infants monitored between January 2012 and June 2015 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
Proposed guidelines for age estimation of infant giant armadillo Priodontes maximus according to infant’s body coloration and behavior.
| Morphology and behaviour of infant | Estimated age of infant |
|---|---|
| Almost all scales in the cephalic shield and carapace light-colored, with only a few slightly darker (“faded”) scales; eyes closed | <20 days |
| Darker scales on the cephalic shield are pale (“faded”) but distinguishable; scales on the carapace remain very pale; eyes closed | ~25–50 days |
| Darker scales of the carapace begin to be distinguishable | ~40 days |
| Eyes closed | <50 days |
| Darker scales of the carapace become easily distinguishable from the light-colored scales; eyes open | ~50 days |
| Darker scales of the carapace and cephalic shield are totally distinguishable for the light-colored ones | >50 days |
| Emerges alone and stays on the sand mound | >5 months |
| Plays with its mother on the sand mound in front of the burrow | >5 months |
| Leaves the burrow to forage alone for a short period (less than 3 h) | ~8–12 months |
| Leaves the burrow to forage alone for over 3 h | >12 months |
| Builds its own burrows | >18 months |
Data based on observations of two infants and one adult female, monitored between January 2012 and June 2015 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
Proposed guidelines for age estimation of the infant giant armadillo Priodontes maximus according to the mother’s behavior.
| Behavior of mother | Estimated age of infant (in weeks) |
|---|---|
| Leaves the nesting burrow for an average of 6 h and returns every night | <3 |
| Leaves the nesting burrow only for one night and then returns | 3–6 |
| Leaves the nesting burrow for only two nights and then returns | 6–10 |
| Almost always closes the burrow entrance when leaving it | <10 |
| Leaves the burrow for more than two nights before returning to the nesting burrow | >10 |
Data based on observations of two infants and one adult female, monitored between January 2012 and June 2015 in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
Behavioral changes in offspring
Offspring behavioral changes can also be used to estimate its age (Tables 2 and 3). Unaccompanied exits from the burrow started to be recorded when offspring were 4 months old. In the first exits, at 4 months of age, infants remained outside, on the sand mound, between 5 and 20 min and then returned in without exploring beyond the sand mound. It was only at 7 months of age that M17 left the burrow on its own for a short trip beyond the sand mound. At 1 year of age, trips lasted an average of 2 h a night (30 min to 3 h). Between 7 and 19 months of age, the time spent away from the burrow gradually increased reaching up to 7 h. M17 was recorded traveling both alone and with F4. It used exclusively burrows dug by its mother until 19 months of age. At 19 months of age, M17 was recorded digging its first burrow alone. Until it started digging its own burrows, M17 and its mother would share a burrow once or twice a week, spending up to 18 h together. Burrow sharing between mother and juvenile was recorded occasionally between the ages of 19 and 22 months, but with lower frequencies than previously observed.
Nesting burrow characteristics
Burrows were always located in non-floodable forested areas, either in murundus, small round-shaped soil mounds, that can be 0.1–3 m high and 1–20 m wide, or in forest strips. Nesting burrows, i.e. burrows that shelter an infant, have large entrances, on average 53 cm wide and 45 cm high (Table 4). The sand mounds in front of nesting burrows are large, when compared to regular burrows. Sand mounds spread for 3 m on average [standard deviation (SD)=0.52] from the entrance of the burrow (maximum length), and are 67.5 cm tall (from soil level to the top of the mound) on average (SD=7.6). One of the characteristics of a nesting burrow is that it is the only burrow to have sand all around the edges of the entrance including on top. This is the result of the mother’s burrow sealing behavior when she leaves the offspring inside.
Comparison between regular burrow entrances (n=87; Desbiez and Kluyber 2013) and nesting burrow entrances (n=7; this study).
| Excavation type | Burrow | Nesting burrow |
|---|---|---|
| Width±SD | 39.68±5.81 | 53.17±5.52 |
| Min–Max | 29–51 | 46–60 |
| Height±SD | 32.64±5.25 | 45±5.36 |
| Min–Max | 26–50 | 41–52 |
Measurements of width and height of burrow entrances in centimeters. Data from 26 giant armadillos (Priodontes maximus) monitored between 2010 and 2017, in the Nhecolândia sub-region of the Brazilian Pantanal (19° 16′ 60″ S, 55° 42′ 60″ W).
SD, Standard deviation.
Discussion
Although this study is mostly based on comprehensive information from one female and brief observations from four others, it does provide the first detailed data on the reproductive behavior of giant armadillos, including gestation period, inter-birth intervals, number of offspring and parental care. Results also allow to estimate the age of giant armadillo offspring according to morphological and behavioral traits (Tables 2 and 3).
There is little reliable information on gestation periods in armadillos. For the nine-banded armadillo (Dasypus novemcinctus), gestation is estimated at 4–5 months, from the moment the fertilized embryo implants (McBee and Baker 1982). Desbiez et al. (2018) observed a 4-month gestation period in the southern naked tailed armadillo (Cabassous unicinctus). Merrett (1983) proposed a 4-month gestation period for giant armadillos. This study suggests a gestation period of 5 months. When F4 was registered with the male M5, she was the only animal being monitored in the study area and was followed very closely through telemetry and camera traps. She was not seen in the proximity of any male besides the registered episode with M5, increasing our confidence that M5 sired her offspring (Supplementary material 1).
Inter-birth intervals are one of the key parameters in determining reproductive rates. There are no reliable estimates for inter-birth intervals for giant armadillos (Carter et al. 2016). The first two of F4’s offspring died before having a chance to disperse. Inter-birth intervals tend to be shortened with the death of a young prior to its independence (Lewison 1998, Bercovitch et al. 2004, Balme and Hunter 2013). The death of the first infant could have shortened the inter-birth period (8 months) when compared to the following birth interval (3 years). At the time of its death, the juvenile M17 was fully weaned and independent, even though it still occupied its mother’s territory and occasional encounters between them were recorded. An inter-birth interval of 3 years is longer than that proposed for the largest South American terrestrial mammal, the lowland tapir (Tapirus terrestris) estimated at 18 months (Medici and Desbiez 2012). This interval is closer to that recorded for the largest terrestrial mammal, the African elephant Loxodonta africana, which ranges between 3.3 and 4.5 years (Gough and Kerley 2006).
The estimated 3-year birth interval is based on a single data point, and more data need to be collected to estimate the inter-birth interval in giant armadillos. To date, observations of births in the study area have been rare, even though other adult females have been monitored. Four adult females have been intensively monitored for up to 2 years during which they did not give birth, nor cared for an infant, nor were they registered interacting with juveniles. This may also indicate that the interbirth period in giant armadillos may be longer than expected, and intervals could potentially be longer than 3 years.
Through camera traps, we also recorded two other females with large-sized young. The juveniles were estimated to be 14–16 months and 16–18 months old, due to their relative size, coloration and behavior. Aya-Cuero et al. (2015) also photographed a female with a large juvenile. These observations confirm the prolonged parental care of the species. Hence, a 3-year inter-birth interval appears to be a reasonable estimate considering the information collected to date here and elsewhere.
Our observations indicate that parental care in giant armadillos is much longer than expected. Neris et al. (2002) estimated that the young suckled for 4–6 months, while Aya-Cuero et al. (2015) predicted an even longer duration. Our monitored infants were completely dependent on its mother’s milk and barely left the burrow until 6–8 months of age. Weaning seems to occur at 11–12 months, when the young begins to forage for short periods, but we suspect juveniles still suckled occasionally as their mother regularly returned for 18-h periods in the burrow with them. In addition, juveniles were dependent on their mother’s burrows until they were 18 months old. Parental care is therefore long in giant armadillos.
Behaviors such as closing the burrow upon departure only occurred when the infants were less than 20 weeks old. The adult female, almost systematically, closed the burrow for the first 12 weeks of the infant’s life, and then gradually abandoned the practice. This behavior was documented for all reproductive events of F4 and was registered by Aya-Cuero et al. (2015) for an infant that was estimated to be 3–4 months old.
Giant armadillo burrows are used by various species (Leite Pitman 2004, Desbiez and Kluyber 2013, Aya-Cuero et al. 2015, Massocato and Desbiez 2017) including carnivores such as ocelots (Leopardus pardalis) and crab-eating foxes (Cerdocyon thous) that could prey on young armadillos. By carefully sealing the entrance, not only by throwing sand with its back paws but also by pressing its upper skull on the sand, compacting it to ensure it is well closed, the female may prevent unwelcome visitors, hide olfactory clues of the infant’s presence, as well as maintain the temperature in the burrow.
It was believed that giant armadillos could have up to two offspring at a time. Most articles cite Krieg (1929) who reports that giant armadillos have one or sometimes two young per year. In addition, there is an old picture that shows a female giant armadillo with two infants. This picture was traced back to Gijzen (1965) who described and published a picture of a female giant armadillo and its two young that arrived together at the Antwerp Zoo on February 8th 1965. However, these two offspring were eventually identified as a different species, the southern naked-tailed armadillo (Cabassous unicinctus) making this picture very misleading (Gijzen 1966, Matthias Papies pers. comm.).
In this study, on all seven occasions where female giant armadillos were recorded with their offspring, they had just one infant. The records of Aya-Cuero et al. (2015) in Colombia also show only one infant with each female. Due to the species’ notoriously low density, and high investment in parental care (this study), it is believed that overall, giant armadillos only have one offspring at a time and twin births would be exceptional.
It is possible that the first infant intensively monitored was the victim of non-parental infanticide – the killing of immature infants by conspecifics other than the parents. Non-parental infanticide has been described for a wide variety of animals in the wild (Hrdy et al. 1994) and in captivity for other armadillo species (Cortés Duarte et al. 2016). Infanticide is a widespread phenomenon among animals and its occurrence has a number of explanations (Hrdy 1979), usually related to the potential benefits to the perpetrator (Ebensperger 1998). The death of the infant could have been accidental following a non-adaptive explanation (Ebensperger 1998). However, the behavior of the male of remaining in the burrow with the infant for 48 h after seemingly “chasing away” the female makes this hypothesis less likely.
The events registered appear to follow the sexual selection hypothesis, in which infanticide is used to obtain increased access to breeding females (Ebensperger 1998). According to Hrdy (1979), the sexual selection hypothesis requires some conditions. (1) Infanticidal males should not kill offspring they have sired. In this case, the male that killed the infant was not the presumed father. (2) Elimination of offspring should shorten the interbirth period of the victimized females. The interbirth interval was much shorter (8 months) after the infanticide, than it was after the natural death of offspring (36 months). (3) Infanticidal males should mate and sire the subsequent offspring of the mother of the infant killed. A genetic sample of the mother, offspring and the infanticidal male were obtained to verify this condition, but await analysis.
Despite a wide distribution, giant armadillos are naturally rare wherever they occur. Density estimates for giant armadillos range from 3.36 individuals/100 km2 (Silveira et al. 2009), 4.7–5.3 individuals/100 km2 (Carter 1983) to 5.77–6.28 individuals/100 km2 (Noss et al. 2004). In the study area, densities were estimated at 7.65 individuals/100 km2 [confidence interval (CI)=5.68–10.19 ind./100 km2] (Desbiez et al. in press). Results on the reproduction of giant armadillos from this study suggest that the population growth rate is very low as only one infant is produced at a time and inter-birth intervals could be at least 3 years. Furthermore, in this study, two of the seven infants recorded in a 7-year period died and two have an unknown fate. As such, the removal of any adult individuals from the population could have a high impact on the local demography. Anthropogenic threats to giant armadillos are typical of Neotropical species and include habitat loss, hunting, road kill and possibly even trade (Anacleto et al. 2014). Due to low population growth rates, the species lacks resilience to anthropogenic impacts, and populations will not recover easily. Reproductive data provided in this paper illustrate one of the reasons giant armadillos are so rare, and why they can so easily become locally extinct. This is currently occurring in more disturbed biomes such as the Atlantic Forest where giant armadillos are heading toward extinction (Srbeck-Araujo et al. 2009).
Acknowledgments
We thank the owners of the Baia das Pedras ranch for their hospitality, generous support and the seven neighboring ranches for their permission to work on their land. We are grateful to Camila Luba and the numerous project trainees for their help in the field. Nina Attias, Anthony B. Rylands and an anonymous reviewer provided helpful comments on the manuscript. This project was part of the Giant Armadillo Conservation Program which benefited from grants mostly from zoos in North America and Europe listed at www.giantarmadillo.org.
References
Abba, A.M. and M. Superina. 2010. The 2009/2010 armadillo Red List assessment. Edentata 11: 135–184.10.5537/020.011.0203Search in Google Scholar
Anacleto, T.C.S. 1997. Dieta e utilização de hábitat do tatu-canastra (Priodontes maximus Kerr, 1792) numa área de cerrado do Brasil Central. Tese de Mestrado, Universidade de Brasília, Brasília.Search in Google Scholar
Anacleto, T.C.S., F. Miranda, I. Medri, E. Cuellar, A.M. Abba and M. Superina. 2014. Priodontes maximus. The IUCN Red List of Threatened Species 2014: Downloaded on 02 November 2016.Search in Google Scholar
Aya-Cuero, C., M. Superina and A. Rodríguez-Bolaños. 2015. Primeros registros de crías de ocarro (Priodontes maximus Kerr, 1792) en Colombia. Edentata 16: 57–64.Search in Google Scholar
Aya-Cuero C., A. Rodríguez-Bolaños and M. Superina. 2017. Population density, activity patterns, and ecological importance of P. maximus (Priodontes maximus) in Colombia. J. Mammal. 98: 770–778.10.1093/jmammal/gyx006Search in Google Scholar
Balme, G.A. and L.T.B. Hunter. 2013. Why leopards commit infanticide. Anim. Behav. 86: 791–799.10.1016/j.anbehav.2013.07.019Search in Google Scholar
Bercovitch, F.B., M.J. Bashaw, C.G. Penny and R.G. Rieches. 2004. Maternal investment in captive giraffes. J. Mammal. 85: 428–431.10.1644/1545-1542(2004)085<0428:MIICG>2.0.CO;2Search in Google Scholar
Carter, T. 1983. The burrows of giant armadillos, Priodontes maximus (Edentata: Dasypodidae). Säugetierkundliche Mitteilungen 31: 47–53.Search in Google Scholar
Carter, T.S. and C.D. Encarnação. 1983. Characteristics and use of burrows by four species of armadillo in Brazil. J. Mammal. 64: 103–108.10.2307/1380755Search in Google Scholar
Carter, T.S., M. Superina and D. Leslie. 2016. Priodontes maximus (Cingulata: Chlamyphoridae). Mamm. Species 48: 21–34.10.1093/mspecies/sew002Search in Google Scholar
Ceresoli, N. and E. Fernandez-Duque. 2012. Size and orientation of giant armadillo burrow entrances (Priodontes maximus) in western Formosa province, Argentina. Edentata 13: 66–68.10.5537/020.013.0109Search in Google Scholar
Cortés Duarte, A., F. Trujillo and M. Superina. 2016. Behavioral responses of three armadillo species (Mammalia: Xenarthra) to an environmental enrichment program in Villavicencio, Colombia. Zoo Biol. 35: 304–312.10.1002/zoo.21305Search in Google Scholar
Desbiez, A.L.J. and D. Kluyber. 2013. The role of giant armadillos as ecosystem engineers. Biotropica 45: 537–540.10.1111/btp.12052Search in Google Scholar
Desbiez, A.L.J., G.F. Massocato, D. Kluyber and R.C.F. Santos. 2018. Unraveling the cryptic life of the southern naked-tailed armadillo, Cabassous unicinctus squamicaudis (Lund, 1845), in a Neotropical wetland: home range, activity pattern, burrow use and reproductive behaviour. Mamm. Biol. 91: 95–103.10.1016/j.mambio.2018.02.006Search in Google Scholar
Desbiez, A.L.J., G.F. Massocato, D. Kluyber, C.N. Luba and N. Attias. 2019. How giant are giant armadillos? The morphometry of giant armadillos (Priodontes maximus) Kerr, 1792) in the Pantanal of Brazil. Mamm. Biol. 95: 9–14.10.1016/j.mambio.2018.12.007Search in Google Scholar
Desbiez, A.L.J., G.F. Massocato, D. Kluyber, L.G.R. Oliveira-Santos and N. Attias. In press. Spatial ecology of the giant armadillo (Priodontes maximus) in Midwestern Brazil. J. Mammal.Search in Google Scholar
Ebensperger, L.A. 1998. Strategies and counterstrategies to infanticide in mammals. Biol. Rev. Cambridge Philos. Soc. 73: 321–346.10.1017/S0006323198005209Search in Google Scholar
Eisenberg, J.F. and K.H. Redford. 1999. Mammals of the Neotropics: the central Neotropics. Ecuador, Peru, Bolívia, Brazil. The University of Chicago Press, Chicago.Search in Google Scholar
Emmons, L.H. and F. Feer. 1997. Neotropical rainforest mammals: a field guide. The University of Chicago Press, Chicago.Search in Google Scholar
Gijzen, A. 1965. Ruzengordeldieren Pridontes giganteus Geoffroy en hun Kleine verwanten. In Onze Zoologische Verzamelingen Zoogdieren. pp. 48–51.Search in Google Scholar
Gijzen, A. 1966. Verrassingen bij de lagere zoogdieren. In Onze Zoologische Verzamelingen Zoogdieren. pp. 9–11.Search in Google Scholar
Gough, K.F. and G.I.H. Kerley. 2006. Demography and population dynamics in the elephants Loxodonta africana of Addo Elephant National Park, South Africa: is there evidence of density dependent regulation? Oryx 40: 434–441.10.1017/S0030605306001189Search in Google Scholar
Hernandez, S.M., D.J. Gammons, N. Gottdenker, M.T. Mengak, L.M. Conner and S.J. Divers. 2010. Technique, safety, and efficacy of intra – abdominal transmitters in nine-banded armadillos. J. Wildlife Manage. 74: 174–180.10.2193/2008-502Search in Google Scholar
Hrdy, S.B. 1979. Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1: 13–40.10.1016/0162-3095(79)90004-9Search in Google Scholar
Hrdy, S.B., C. Janson and C. van Schaik. 1994. Infanticide: let’s not throw out the baby with the bath water. Evol. Anthropol. 3: 151–154.10.1002/evan.1360030503Search in Google Scholar
Krieg, H. 1929. Biologische Reisestudien in Südamerika. IX: Gürteltiere. Zeitschrift für Morphologie und Oekologie der Tiere 14: 166–190.10.1007/BF00419346Search in Google Scholar
Kluyber, D. 2016. Avaliação da Prevalência de Patógenos Zoonóticos de Importância para Saúde Pública em Tatus de Vida Livre – Mato Grosso do Sul – Brasil. Master’s thesis. Universidade de São Paulo, USP. pp. 142.Search in Google Scholar
Leite Pitman, R., G. Powell, D. Cruz, M. Escobedo, K. Escobar, V. Vilca and A. Mendoza. 2004. Habitat use and activity of the giant armadillo (Priodontes maximus): preliminary data from southeastern Peru. Presented at the Society for Conservation Biology Meeting, New York.Search in Google Scholar
Lewison, R. 1998. Infanticide in the hippopotamus: evidence for polygynous ungulates. Ethol. Ecol. Evol. 10: 277–286.10.1080/08927014.1998.9522857Search in Google Scholar
Massocato G.F. and A.L.J. Desbiez. 2017. Presença e importância do tatu-canastra, Priodontes maximus (Kerr, 1792), na maior área protegida do leste do Estado do Mato Grosso do Sul, Brasil. Edentata, Washington, D.C., 18: 26–33.10.2305/IUCN.CH.2017.Edentata-18-1.4.enSearch in Google Scholar
McBee, K. and R.J. Baker. 1982. Dasypus novemcinctus. Mamm. Species 162: 1–9.10.2307/3503864Search in Google Scholar
Medici, E.P. and A.L.J. Desbiez. 2012. Population viability analysis (PVA): using a modeling tool to assess the viability of tapir populations in fragmented landscapes. Integrative Zool. 7: 356–372.10.1111/j.1749-4877.2012.00318.xSearch in Google Scholar PubMed
Meritt, D.A. Jr. 2006. Research questions on the behavior and ecology of the giant armadillo (Priodontes maximus). Edentata 7: 30–33.10.1896/1413-4411.7.1.30Search in Google Scholar
Merrett, P.K. 1983. Edentates: project for city and guilds animal management course. Zoological Trust of Guernsey, Guernsey, UK.Search in Google Scholar
Neris, N., F. Colman, E. Ovelar, N. Sukigara and N. Ishii. 2002. Guía de mamíferos medianos y grandes del Paraguay: distribución, tendencia poblacional y utilización. Secretaría del Ambiente (SEAM), Asunción, Paraguay.Search in Google Scholar
Noss, A.J., R. Peña and D.I. Rumiz. 2004. Camera trapping Priodontes maximus in the dry forests of Santa Cruz, Bolivia. Endanger. Species Update 21: 43–52.Search in Google Scholar
Porfirio, G.E.O., P. Sarmento, N.L.X. Filho, S.P. Da Silva Leal, V.F. Moreira, F.A. Rabelo, J. Cruz and C. Fonseca. 2012. New records of Giant Armadillo Priodontes maximus (Cingulata, Dasypodidae) at Serra do Amolar, Pantanal of Brazil. Edentata 13: 72–75.10.5537/020.013.0110Search in Google Scholar
Samuel, M.D. and M.R. Fuller. 1994. “Wildlife radiotelemetry.” In Research and management techniques for wildlife and habitats. Wildlife Society, Bethesda, Maryland, USA. Edited by: Bookhout, T.A. pp. 370–418.Search in Google Scholar
Sibly, R.M. and J. Hone. 2002. Population growth rate and its determinants: an overview. Philos. Trans. R. Soc. London 357: 1153–1170.10.1017/CBO9780511615740.002Search in Google Scholar
Silveira, L., A.T. Jácomo, M.M. Furtado, N.M. Torres, R. Sollmann and C. Vynne. 2009. Ecology of the giant armadillo (Priodontes maximus) in the grassland of Central Brazil. Edentata 8–10: 25–34.10.1896/020.010.0112Search in Google Scholar
Soriano, B.M.A. 2000. Boletim Agrometeorológico: Fazenda Nhumirim. Embrapa Pantanal. Boletim Agrometeorológico 4. pp. 81.Search in Google Scholar
Srbek-Araujo, A.C., L.M. Scoss, A. Hirsch and A.G. Chiarello. 2009. Records of the giant-armadillo Priodontes maximus (Cingulata: Dasypodidae) in the Atlantic Forest: are Minas Gerais and Espírito Santo the last strongholds of the species? Zoologia 26: 461–468.10.1590/S1984-46702009000300010Search in Google Scholar
Superina, M., N. Pagnutti and A. M. Abba. 2014. What do we know about armadillos? An analysis of four centuries of knowledge about a group of South American mammals, with emphasis on their conservation. Mammal Rev. 44: 69–80.10.1111/mam.12010Search in Google Scholar
Walsh, J. and R. Gannon. 1967. Time is short and the water rises, Operation Gwamba: the story of the rescue of 10,000 animals from certain death in a South American rain forest. e. P. Dutton and Co., Inc., New York.Search in Google Scholar
ZIMS. 2017. Giant armadillo (Priodontes maximus) species historical holdings. 27 March 2017. Zoological Information Management System, Species360: Bloomington, MN, USA.Search in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/mammalia-2019-0018).
©2020 Walter de Gruyter GmbH, Berlin/Boston

