This electronic version has been made freely available under a Creative Commons (CC-BY-NC-ND) license. A copy of the license can be viewed at http://creativecommons.org/licenses/by-nc-nd/2.0/.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
In this chapter, we review the etiology and pathogenesis of Type 1 diabetes mellitus (T1DM), with particular emphasis on the most common immune mediated form. Whereas Type 2 diabetes (T2DM) appears to be an increasing price paid for worldwide societal affluence, there is also evidence worldwide of a rising tide of T1DM. The increase in understanding of the pathogenesis of T1DM has made it possible to consider interventions to slow the autoimmune disease process in an attempt to delay or even prevent the onset or slow the progression of hyperglycemia. Although the prevention of T1DM is still at the stage of research trials, the trials are often mentioned in the lay press. Current investigations will determine if antigen-based therapies can in fact abrogate ongoing autoimmunity via immuno-stimulation and ultimately prevent diabetes in humans without the risks of general immunosuppression. We also review the etiology and pathogenesis of T2DM and monogenic forms of diabetes that may be confused with T1DM or T2DM. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Diabetes Mellitus (DM) is a syndrome of disturbed metabolism involving carbohydrate, protein, and fat which results from the degree of insulin deficiency (absolute or relative) and tissue sensitivity to its actions. The combination(s) of insulin deficiency and sensitivity to its actions bring about distinct clinical phenotypes with varying severity of disturbed metabolism, most conveniently monitored by the degree of hyperglycemia. Absolute insulin deficiency (Type 1 DM) occurs with autoimmune destruction of insulin secreting β-cells (Type 1A DM) and other congenital (genetic defects in the formation or function of the endocrine pancreas), or acquired (relapsing pancreatitis and pancreatectomy) conditions. Absolute deficiency of insulin action also can occur in the total absence of insulin receptors, a rare event. Relative insulin deficiency occurs with genetic or acquired defects in insulin synthesis or secretion that are inadequate to overcome the resistance caused by fewer functioning insulin receptors, or resistance to insulin action induced by stress, drugs, and most commonly obesity (Type 2 DM).The acute clinical manifestations are those related to hyperglycemia which exceeds renal threshold to result in polyuria, increased thirst, dehydration, electrolyte disturbances, weight loss, and metabolic decompensation, in extreme degree known as diabetic ketoacidosis and non-ketotic hyperosmolar coma. The chronic complications include macrovascular (CAD, CVD, amputations) and microvascular (retinopathy, nephropathy, neuropathy) lesions. Both the acute and chronic complications are inversely related to the degree of metabolic control achieved. These brief introductory comments form the basis for the etiology, pathogenesis, classification and diagnosis of diabetes mellitus.
Classification and Diagnosis of Diabetes
The American Diabetes Association Standards of Medical Care for Diabetes 2021(1) proposes the following classification (Table 1).
Table 1.
Classification of Diabetes
| Type 1 Diabetes owing to autoimmune destruction of insulin secreting β-cells leading to insulin deficiency |
| Type 2 Diabetes owing to inadequate insulin secretion that cannot overcome the existing degree of insulin resistance |
| Gestational diabetes (diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes) |
Diabetes owing to other causes
|
Criteria for the Diagnosis of Diabetes Mellitus
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus recommends the following criteria for diagnosing DM (1). Two replicate fasting glucose levels that exceed 126 mg/dl (>7 mmol/L) is consistent with diabetes even in the absence of symptoms. Normal fasting blood glucose levels of 100 mg/dl or above are considered impaired fasting glucose (IFG). Persons with IFG levels (FPG= 100-125 mg/dl (5.66.9 mmol/l) and/or with impaired glucose tolerance test (IGT) (2hour post-load glucose 140-199 mg/dl (78.8 mmol/L-11.1 mmol/L) are at risk of diabetes and should be observed periodically to detect hyperglycemic progression. Replicate, two-hour glycemic responses >200 mg/dl (>11.1 mmol/L) after a standard oral glucose tolerance test also indicate diabetes. This stage is often reached before the fasting glucose levels rise in T2DM and post-prandial hyperglycemia may precede fasting hyperglycemia by months to years. The reliance on only fasting glucose levels is generally more useful for identification of impending T1D but not for T2D.
The ADA now recommends that measurement of HbA1c levels can be used in clinical practice for the diagnosis of diabetes, since the onset is seldom so acute that it will not be reflected in elevated HbA1c levels Table 2 (1).
Table 2.
| Stage | Latent | Impaired glucose tolerance | Diabetes |
|---|---|---|---|
| Diagnostic criteria | Presence of 2 or more autoantibodies AND Normal glucose levels | Fasting plasma glucose: 100-125 mg/dl OR 2hour plasma glucose during OGTT*: 140-199 mg/dl OR HbA1C+: 5.7-6.4% | Fasting plasma glucose: ≥126 mg/dL OR 2hour plasma glucose during OGTT*: ≥200 mg/dl OR Random plasma glucose: ≥200 mg/dl with symptoms of polyuria, and weight loss. OR HbA1C+ ≥6.5%. |
- *
The OGTT should be performed as described by the World Health Organization (1.75 gm/kg up to 75 gm, using a glucose load containing anhydrous glucose dissolved in water).
ETIOLOGIC CLASSIFICATION
Type 1 Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) comprises several diseases of the pancreatic ß cells which lead to an absolute insulin deficiency. This is usually considered to be the result of an autoimmune destruction of the pancreatic ß cells (type 1A). Some patients with T1DM with no evidence of ß cell autoimmunity have underlying defects in insulin secretion often from inherited defects in pancreatic ß cell glucose sensing and from other genetic or acquired diseases.
Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is by far the more common type of diabetes and is characterized by insulin resistance resulting from defects in the action of insulin on its target tissues (muscle, liver, and fat), but complicated by varying and usually progressive failure of beta cells’ insulin secretary capacity. Most patients with T2DM in the US and Europe are overweight or obese, however in India and China, most T2DM patients have a lean body mass index (BMI), albeit with increased visceral and hepatic fat.
Monogenic Diabetes
Monogenic forms of diabetes are characterized by impaired secretion of insulin from pancreatic β cells caused by a single gene mutation. These forms comprise a genetically heterogenous group of diabetes including, maturity onset diabetes of the young (MODY), permanent or transient neonatal diabetes, and mitochondrial diabetes. MODY is the most common form of monogenic diabetes, with autosomal dominant transmission of one of several genes encoding a primary defect in insulin secretion.
TYPE 1 DIABETES MELLITUS
Epidemiology of Type 1 Diabetes
T1DM is one of the most common chronic diseases of childhood and is classified as an autoimmune disease. Most common autoimmune disorders predominantly affect females, but, T1DM equally affects males and females with a slight male predominance in younger children. This and other inconsistencies have raised questions as to whether T1DM is a “pure” auto-immune disease or whether the auto-immune component is a marker of a separate primary trigger (3,4). We discuss these issues later in this chapter.
The incidence and prevalence of T1DM vary by age, season, geographic location, and within different racial and ethnic groups. Of cases diagnosed before the age of 20, however, two peaks of T1DM presentation are observed; one between 5 and 7 years of age, and the other during puberty at the mid-teens (5). However, first presentation of T1DM actually is as common in adulthood as it is in childhood and is characterized by a milder course in adults; the term LADA, (Latent, Auto-immune, Diabetes of Adults) is used to describe this entity. A seasonal variation in the incidence of T1DM is also observed; the majority of new cases of T1DM are diagnosed mostly in autumn and winter (6). Findings from large T1DM registry studies such as the World Health Organization Multinational Project for Childhood Diabetes, known as the DIAMOND Project, EURODIAB and others monitor incidence and other epidemiological markers.
The World Health Organization Multinational Project for Childhood Diabetes, known as the DIAMOND Project (in 50 countries), EURODIAB (in Europe), and SEARCH for Diabetes in Youth (in the USA) were established to address the implications of diabetes in youth and describe the incidence of T1DM. Wide variations in incidence of T1DM exist throughout the world, lowest in China and Venezuela (0.1 per 100,000 per year) and highest in Finland and Sardinia (50-60 per 100,000 per year) (7). A multicenter study focusing on identifying the prevalence and incidence of diabetes by type, age, gender, and ethnicity found a 1.8% annual increase in the prevalence of T1DM among American youth from 2002-2003 to 2011-2012, whereas T2DM had increased 4.8% annually from 2002-2003 to 2011-2012 (Table 3) (8). The greatest increase was seen in youth of minority racial/ethnic groups (8). Similar rates of increase in T2DM in teens are reported from the UK, India, China and Japan.
Table 3.
Incidence of T1DM in the USA (per 100,000/year)
| Age Group | ||||
|---|---|---|---|---|
| 0-4 yr | 5-9 yr | 10-14 yr | 15-19 yr | |
| Non-Hispanic White | 18.6 | 28.1 | 32.9 | 15.1 |
| African American | 9.7 | 16.2 | 19.2 | 11.1 |
| Hispanic American | 9.1 | 15.7 | 17.6 | 12.1 |
| American Indian | 4.1 | 5.5 | 7.1 | 4.8 |
| Asian and Pacific Islander American | 6.1 | 8.0 | 8.3 | 6.8 |
| All | 14.3 | 22.1 | 25.9 | 33.1 |
Although, there is a wide variance in the incidence and prevalence of diabetes throughout the world, the number of youths who are being diagnosed with T1DM has been growing at an annual rate of about 3 percent (9) and a similar increased annual rate was also observed among U.S. youth (10). This rising incidence of T1DM in children across the world in a short period of time clearly cannot be explained by genetic factors. Analytical epidemiological studies suggest that environmental risk factors, operating early in life, might be contributing to the increasing trend in incidence of T1DM (11,12).
On the basis of estimates for the number of people with diabetes in 2014, the cost of health care of diabetes in the US is estimated to be $105 billion per annum and the direct annual cost of diabetes in the world is $825 billion (13). However studies indicate that many more diabetic adults diagnosed as having T2DM phenotype actually have T1DM as defined by the presence of antibodies to islet cell components (14,15); the term LADA, Latent Autoimmune Diabetes of Adults, is often used to describe this group (16).
Natural History of Type 1 Diabetes
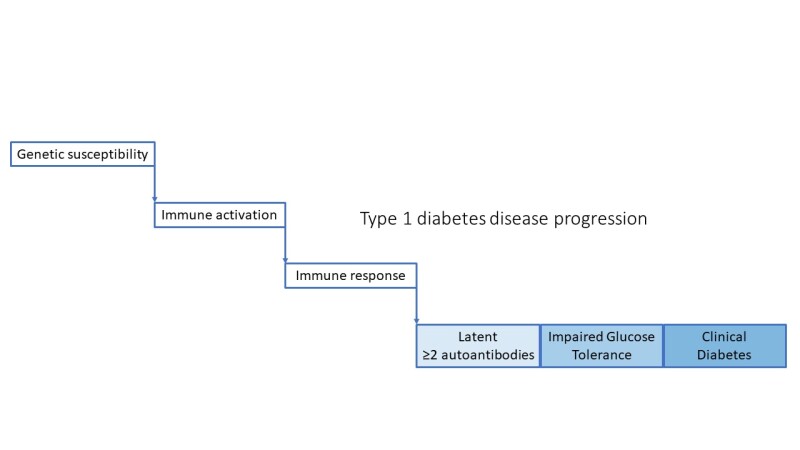
After immune activation in the setting of genetic susceptibility, the disease progresses through pre-symptomatic stages identified by presence of autoantibodies and impaired glucose intolerance, arising from further loss of β-cell function and ultimately resulting in clinical diabetes. (Figure 1)
Structure and Functions of the Pancreas
Pancreatic ß cells secrete insulin and are found in the islets of Langerhans. These islets are specialized groups of a few hundred to a few thousand endocrine cells that are anatomically and functionally discrete from pancreatic exocrine tissue, the primary function of which is to secrete pancreatic enzymes into the duodenum. Normal subjects have about one million islets, which in total weigh only 1-2 grams and constitute less than 1% of the mass of the pancreas. Furthermore, islets are composed of various types of cells that are interconnected as a regulatory network to regulate the disposition of nutrients and their utilization for energy use and tissue growth and repair. At least 70% are ß cells localized in the core of the islets, surrounded by α-cells that secrete glucagon, δ-cells that secrete somatostatin, and PP cells that secrete pancreatic polypeptide. All the cells communicate with each other through their extracellular spaces and through gap junctions; communication is further modulated by a rich network of sympathetic and para sympathetic innervation.
Insulin, a peptide hormone composed of 51 amino acids is synthesized, packaged and secreted in pancreatic ß cells. Insulin is synthesized as preproinsulin in the ribosomes of rough endoplasmic reticulum. The preproinsulin is then cleaved to proinsulin that is transported to the Golgi apparatus where it is packaged into secretory granules. Most of the proinsulin is cleaved into equimolar amounts of insulin and connecting (or C)-peptide in the secretory granules. Because the C-peptide sequence differs from that of insulin, and because, unlike insulin, it is not extracted by the liver, it is possible to estimate β-cell insulin secretion by measuring C-peptide, even in the presence of insulin antibodies resulting from insulin replacement therapy that impair the ability to measure insulin directly. Similarly, because C-peptide is an index of endogenous insulin secretion, and because C-peptide is not extracted by the liver, the ratio of C-peptide: insulin should exceed 1; when it is less than 1, implying a high insulin value, exogenous insulin may have been used. This has diagnostic and forensic utility in diagnosing causes of hypoglycemia.
Glucose is a major regulator of insulin secretion (Figure 2). When extracellular fluid glucose concentrations rise after a meal, glucose is taken up by the ß cells via glucose transporters, GLUT2 and GLUT1. Glucose is then phosphorylated into glucose-6-phosphate by islet specific glucokinase and metabolized, thereby increasing cellular ATP concentrations. The rise in ATP raises the resting ratio of ATP:ADP, that closes ATP dependent potassium channels (K-ATP) in the β-cell membrane, resulting in accumulation of intracellular potassium, causing membrane depolarization and influx of calcium via a voltage gated calcium channel. The rise in intracellular free calcium in ß-cells promotes margination of the secretory granules, their fusion with the cell membrane, and release of cell contents which include insulin into the extracellular space. An immediately releasable pool of insulin granules adjacent to the plasma membrane is responsible for an acute (first phase) insulin response; with ongoing stimulation, a pool of granules in the interior of the cell is mobilized and released as the “second phase” response. Amino acids also stimulate insulin release by a similar mechanism that involves the enzyme glutamate dehydrogenase which enables metabolism and ATP production by certain amino acids. Defects in the genes regulating these processes may result in diabetes if the K-ATP channel is prevented from closing normally (activating mutations) or syndromes of hyperinsulinemic hypoglycemia if the K-ATP channel is prevented from opening (inactivating mutations). These aspects are discussed in greater detail in the section on Monogenic forms of diabetes (see below).
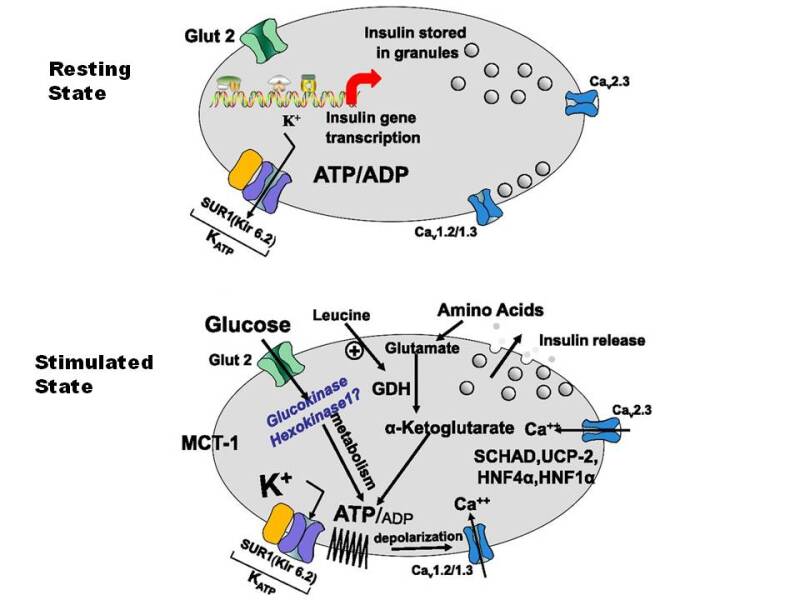
Figure 2.
Insulin secretion by Pancreatic β cells. In the stimulated state, glucose is transported into the β cell by the GLUT2 transporter which undergoes phosphorylation by glucokinase and glucose is then metabolized. This results in an increase in the ATP/ADP ratio and initiation of a cascade of events that is characterized by closure of the K-ATP channel, decreased flux of potassium across the membrane, membrane depolarization, and calcium influx. This cascade ultimately results in insulin release from storage granules. The K-ATP channel shown is composed of four small subunits, Kir6.2, that surround a central pore and four larger regulatory subunits constituting SUR1. In the resting state, the potassium channel is open, modulated by the ratio of ATP to ADP. Leucine also stimulates insulin secretion by allosterically activating GDH and by increasing the oxidation of glutamate; this then increases the ATPADP ratio leading to the cascade of events beginning with closure of the KATP channel.
MCT-1: Monocarboxylate transporter-1, SCHAD: Short chain 3-hydroxyacyl-CoA dehydrogenase, SUR1: Sulfonylurea receptor 1, Kir 6.2: Potassium Inward Rectifying Channel 6.2, UCP-2: Uncoupling protein 2, HNF4α: Hepatocyte Nuclear Factor 4α, HNF1 α: Hepatocyte Nuclear Factor 4α, K+: Potassium, ATP: Adenosine Triphosphate, GDH: Glutamate Dehydrogenase, GLUT-2: Glucose Transporter 2
Metabolic Derangements of Type 1 Diabetes
As the pancreatic ß cell mass declines in an islet cell antibody (ICA) positive person, the first metabolic abnormality discernable is a decline in the first phase of insulin release (FPIR) to an IVGTT (18). The insulin level after a 3-4 minute infusion of glucose at 0.5Gms/kg rises abruptly in normal children at about 8 years of age, perhaps coincident with the onset of adrenarche (19). In the relatives and children from the general population with positive ICA, a decline in the FPIR is a strong predictive marker of evolving diabetes (19-21).
Subsequently, in evolving T1DM there is a rise in the fasting glucose level followed by an inability to keep the two-hour, post-OGTT glucose level below 200mg/dl (11.1mM). Transient insulin resistance also occurs in untreated T1DM and is due to raised levels of free fatty acids (FFAs) from uncontrolled lipolysis (22), as well as decreased levels of hepatic glucokinase and insulin regulated GLUT 4 glucose transporters in adipocytes which contribute to the onset of symptomatic diabetes (23-25). Prolonged hyperglycemia itself likely impairs the ability to secrete insulin and when insulin replacement therapy begins, there is usually some recovery in the patient's ability to secrete insulin (the "honeymoon" period). However, within months to years, this partial recovery in endogenous insulin secretion ultimately fails. If it does not fail after 2 years, another form of diabetes, such as MODY should be suspected. Initially, the glucagon secreting cells within the pancreatic islets remain relatively preserved, resulting in excessive secretion of glucagon relative to insulin after protein meals (26). These elevated glucagon levels exacerbate the effects of the insulin deficiency, and promote lipolysis and ketogenesis, effects that can be partially reversed by an infusion of somatostatin (27). As the mass of islet cells decline, there is also loss of amylin, an islet cell hormone that down-regulates glucagon secretion. Thus, an analogue of amylin (pramlintide- marketed under the trade name Symlin) can be used as adjunctive therapy with insulin replacement. In time, with continued loss of islets, glucagon deficiency develops in established long standing T1DM, rendering patients more susceptible to insulin-induced hypoglycemia (26,28).
Insulin is the hormone of "feasting", promoting utilization and deposition of ingested nutrients into body stores, as well as having multiple anabolic effects in many tissues. Progressive insulin deficiency thus induces a starvation like state, associated with excessive hepatic and renal gluconeogenesis, decreased peripheral utilization of glucose, hyperglycemia with resultant glycosuria, loss of water and sodium salts, and proteolysis in muscle liberating amino acids such as alanine and glutamine as substrates for gluconeogenesis (29-31). Uncontrolled lipolysis leads to the rapid mobilization of fatty acids from adipose tissue and the increased delivery of fatty acids to the liver leading to the increased synthesis of triglycerides and secretion of very low-density lipoprotein (VLDL).
With severe insulin deficiency the fatty acids delivered to the liver are metabolized to yield beta hydroxybutyric and aceto-acetic acids (ketone bodies) and contribute to keto-acidosis. Ketoacidosis is a life-threatening metabolic decompensation that is characterized by hyperglycemia, dehydration, metabolic acidosis and ketosis, all the result of the effects of severe insulin deficiency as well as the counter-regulatory stress hormones, cortisol, growth hormone, catecholamines and glucagon. Specifically, hepatic glucokinase levels fall with insulinopenia, synthesis of hepatic triglyceride and glycogen levels decline, malonyl CoA falls and thereby carnitine palmitoyl transferase-I levels rise promoting the transport of fatty acyl-CoA into mitochondria with the formation of acetyl-CoA (32-34). In the liver, acetyl-CoA is converted into ß-hydroxybutyrate and acetoacetate in a proportion that depends upon the prevailing redox state, which provide an additional fuel substrates for muscle and brain (31,35,36). Lipoprotein lipases are also inactivated, leading to reduced hydrolysis of triglycerides that, if severe, may turn the serum milky with increased VLDL characteristic of the type 4 lipemic phenotype (37-39).
Genetic Susceptibility to Type 1 Diabetes
Individuals with autoimmune T1DM have inherited a number of quantitative trait loci (QTL) that encode protective and predisposing alleles which have exceeded the net genetic threshold required to predispose them to the disease (40). However, this genetic threshold (penetrance) is dependent in turn on chance interactions with greater predisposing than protective environmental forces. The multiple genetic influences in T1DM comprise a major effect from DR/DQ genotypes of the HLA complex (some 50% of the genetic effect), coupled to several other QTLs with minor influences (Table 4). All of the latter QTLs are not obligatory genetic elements themselves since they are of minor-influence, but they collectively interact to create additive influences on the genetic threshold. Siblings of a diabetic patient develop T1DM at about 15-fold greater frequency than persons in the general population (prevalence 1:250-300), vs. a value of 15. The HLA predisposition to T1DM is encoded by cis- and trans complementation DQA1*/DQB1* heterodimers which have an arginine at residue 52 of the A chain and a neutral amino acid (DQB1*0302, *0201) rather than a charged aspartic acid at residue 57 of the B chain (DQB1*0602/3 and DQB1*0301) (40), as modified by DRB1*04 subtypes (*0401 and *0405 are susceptible and *0403 and 6 are resistant types) (41) in the HLA genotype. Further, HLA-DP alleles have also been implicated, even though they are at a considerable recombination frequency away from the closely linked DR/DQ loci (42). Other genes involved include the variable number of tandem repeat (VNTR) alleles 5' to the insulin (INS) gene on chromosome 11p15, where the protective class III alleles (>200 repeats) are associated with increased expression of insulin in the thymus, leading to a more efficient eradication of insulin autoreactive T cells than class I alleles (26-63 repeats) that confer susceptibility to develop diabetes (43,44). There are also CTLA-4 gene polymorphisms on chromosome 2q that are associated with T1DM. CTLA-4 is an induced accessory molecule that is expressed on activated T cells. CTLA-4 interacts with B7.2 expressed by antigen presenting cells (APC), signaling apoptosis of T cells that become activated as part of an immune response, thereby confining the immune response. The non-obese diabetic (NOD) mouse, a model for autoimmune diabetes, has an enlarged lymphoid mass because of resistance of their T cells to undergo apoptosis, as do CTLA-4 knockout mice, which readily develop lymphocytic organ infiltrates like NOD mice. These genes thus collectively affect the general ability to be tolerant to "self" antigens. Another susceptibility locus, (the IDDM 4) in the genomic interval on chromosome 11q13harbors the high affinity IgE Fc receptor gene that has been linked to atopy and asthma, which are characterized byTh2 responses that may protect individuals against the development of anti- islet Th1 responses, and thereby protect against T1DM. There are other genomic intervals associated with or linked to T1DM that have been putatively mapped, but these mostly lack plausible candidate genes in the DNA region, and pathogenic mechanisms for them cannot yet be offered. The NOD mouse however has been subjected to extensive genetic mapping studies, in the hopes that genomic intervals harboring susceptibility or protective genes which are syntenic to humans will be discovered, thus hastening the identification of equivalent defective genes.
Table 4.
Genotypes of the HLA Complex Associated with Diabetes Mellitus
| Locus | Chromosome | Candidate Genes/Microsatellites | References |
|---|---|---|---|
| IDDM1 | 6p21.3* | HLA-DQ/DR | (45,46) |
| IDDM2 | 11p15* | INS VNTR | (47,48) |
| IDDM3 | 15q26 | D15s107 | (49) |
| IDDM4 | 11q13 | MDU1, ZFM1, RT6, FADD/MORT1, LRP5 | (50,51) |
| IDDM5 | 6q24-27 | ESR, MnSOD | (52) |
| IDDM6 | 18q12-q21 | D18s487, D18s64, JK (Kidd locus) | (53) |
| IDDM7 | 2q31 | D2s152, IL-1, NEUROD, GALNT3 | (54) |
| IDDM8 | 6q25-27 | D6s264, D6s446, D6s281 | (52) |
| IDDM9 | 3q21-25 | D3s1303 | (55) |
| IDDM10 | 10p11-q11 | D10s193, D10s208, D10s588 | (56) |
| IDDM11 | 14q24.3-q31 | D14s67 | (57) |
| IDDM12 | 2q33* | CTLA-4, CD28 | (58) |
| IDDM13 | 2q34 | D2s137, D2s164, IGFBP2, IGFBP5 | (59) |
| IDDM14 | ? | NCBI# 3413 | |
| IDDM15 | 6q21 | D6s283, D6s434, D6s1580 | (52) |
| IDDM16 | ? | NCBI# 3415 | |
| IDDM17 | 10q25 | D10s1750- D10s1773 | (60) |
| 2p12 | EIF2AK3 | (61) | |
| 5p11-q13 | (62) | ||
| 16p | D16s405- D16s207 | (62) | |
| 16q22-q24 | D16s515- D16s520 | (55) | |
| 1q42 | D1s1617 | (63) | |
| Xp11 | DXS1068 | (64) |
In summary, T1DM is a complex, multifactorial disease involving genetic predisposition and an environmental triggering event, of which viral causes have been proposed. Although more than 50 loci have been identified, genes involved in immune regulation including HLA subtypes, VNTR in insulin itself, CTLA4, PTPN22, AIRE, and IL2R remain most prominent (65,66). The HLA association, especially class II, remains the strongest predictor of T1DM risk. The heterozygous DR3/DR4 genotype carries the highest genetic risk for T1DM in non-Hispanic whites (45-70). In conclusion, insulin expressing islets from recent-onset T1D subjects show overexpression of interferon stimulated genes (ISGs), with an expression pattern similar to that seen in islets infected with virus or exposed to IFN-γ/interleukin-1β or IFN-α.
Autoantigens and Autoantibodies in Type 1 Diabetes
The Doniach group in London, first reported islet cell autoantibodies in patients with autoimmune polyglandular syndromes (APSs) (71), especially in those with APS type-1 (APS-1) (72), even though such patients did not often develop diabetes. Lendrum and colleagues, having failed to find serological evidence for an autoimmune basis for chronic pancreatitis, did succeed in finding Islet Cell Antibodies (ICA) detectable by indirect immunofluorescence in patients with T1DM. Islet cell surface reactive autoantibodies and autoreactive peripheral blood T cells were also reported (73,74). Over the years that followed, the presence of ICA in US patients was confirmed but with distinctly lower frequencies of ICA among African American diabetic patients (75). Insulin autoantibodies (IAA) were discovered in patients with T1DM before their first dose of insulin replacement had been received (76). The presence of IAA together with ICA identified a group of non-diabetic relatives of probands with T1DM, that were at high risk for T1DM themselves (77). Insulin itself is not an ICA antigen that can be detected by the indirect immunofluorescent technique. Subsequently, much of the antigenic nature of the ICA reactivity has become clearer. It was recognized that many patients with "stiff" man syndrome who were prone to develop diabetes, also had ICA and autoantibodies to glutamic acid decarboxylase (GAD65). These GAD autoantibodies penetrated the blood brain barrier. High concentrations of GAD in the cerebellum reduce brain levels of the inhibitory neurotransmitter gamma aminobutyric acid (GABA), thereby causing the appearance of temporal lobe epilepsy, depressed cognition, muscle spasms, cerebellar incoordination and motor dysfunctions. That GAD65 was the antigen that accounted for the 64 KDa islet cell protein previously discovered by Baekkeskov to react with autoantibodies in T1DM, was later confirmed by the same investigator (78). Antibodies to recombinant GAD65 and GAD67 in T1DM patients were soon reported (79). The autoantibodies reacted to the antigens by conformational rather than linear epitopes, and thus with native rather than denatured antigens. Therefore, they were best detected by liquid phase assays such as radioimmunoassay, rather than by an ELISA technique. In stiff-man syndrome, the predominant GAD autoantibodies reacted with linear epitopes. It became known that besides islet cell 64 KDa sized proteins, autoantibodies in the sera of T1DM patients also precipitated islet cell proteins of 50, 40 and 37 KDa as well (80).
The next islet cell antigen discovered was one of the two-dozen tyrosine phosphatases expressed in islet cells, insulinoma antigen-2 (IA-2) (81). This antigen shared structural homologies with the ICA-512 antigen (82). A second tyrosine phosphatase named IA-2ß was discovered next (83). These additional tyrosine phosphatase antigens allowed for the matching of the islet cell proteins previously identifiable only by their molecular weights. Thus, GAD65 and its tryptic fragment explained the 64 and 50 KDa proteins, while tryptic fragments of IA-2 and IA- 2ß were identical with the 40 KDa and the 37 KDa islet precipitable proteins respectively (84). The tyrosine phosphatases are a family of transmembrane enzymes of which only these two are expressed by the pancreatic islets and react with T1DM autoantibodies. The reactivity is almost exclusively with the internal domains of these molecules, suggesting that they arise as a consequence of islet cell damage from autoimmunity. Antibodies to IA-2 cross-react with those of IA-2ß in about 50% of the patient sera. Some unusual patient sera however react exclusively with IA-2ß. The question of why only these two members of the tyrosine phosphatase family are targets of islet cell autoimmunity has been answered by the finding that they are relatively resistant to proteolytic enzymatic digestion, and once released from islet cells after their lysis, are insoluble and thus become better antigens for auto-immunization, than those that remain soluble and are more rapidly digested (85).
Recently, another antigen of 38KDa size (GLIMA) was added to the islet cell group, albeit only a minority of patient's sera reacts to it (86). Still more islet cell autoantigens are likely to be discovered. The detection of islet cell autoantibodies is useful for differentiating T1DM from diabetes of other causes, and can be used to predict onset of diabetes months to years before onset of the clinical disease (20,21,87,88) in non-diabetic relatives of probands with T1DM. Importantly, the clinical onset of the disease is often long preceded by the appearance of autoantibodies reactive to islet cells (ICA) (88) and to insulin (77), as independent age-related variables in predicting a diabetic outcome (89). Islet cell autoantibodies (ICA) also show a strong tendency to disappear after diabetes onset when all ß cells are destroyed (90,91).
Studies in mice demonstrated a critical role of autoantibodies to GAD65 in the induction of autoimmune diabetes in NOD mice. In humans, the German BABY-DIAB study and the Finnish TRIGR study showed that islet autoantibodies which are mostly IgG class can be transferred through the placenta from islet antibody-positive mothers to their offspring (92,93). Most of the antibodies, however, disappeared from the circulation of the infant within the first year of life, indicating that they represent maternal antibodies and unlikely that they are markers of fetal induction of B-cell autoimmunity (93). In the German BABY-DIAB study, it was demonstrated that 729 offspring of mothers with T1DM had significantly lower risk of developing multiple islet autoantibodies (5 year risk 1.3%) and diabetes (8-year risk 1.1%) when they were GAD or IA-2 positive, than offspring who were islet autoantibody negative at birth (94). These findings suggest that fetal exposure to islet autoantibodies may protect from future diabetes. Furthermore, the German BABY-DIAB study finding is consistent with the overall decreased risk of development of diabetes in offspring of mother with T1DM compared with that of offspring of fathers with T1DM and nondiabetic mothers (95).
The timing of the appearance of the autoantibodies seems to be important. It was found that progression to multiple islet autoantibodies was fastest in children who were antibody positive by age 2 years and that progression to diabetes was inversely related to the age of first positivity for multiple autoantibodies (96).
The presence of multiple autoantibodies strikingly increases the risk of diabetes, whereas one of the above autoantibodies in the absence of all of the others when tested for, denotes only a modestly increased risk (20,21). This suggests that antigenic epitope spreading is involved in a sustained or accelerated autoimmune attack (72) (97). Besides autoimmunity to islet cell autoantigens, patients with T1DM are subject to other autoimmunities. Thus T1DM is a component part of the autoimmune polyglandular syndromes, commonly in APS-2 (Diabetes Mellitus, Addison Disease, Hypothyroidism) and with less frequency in APS-1(AIRE gene mutations) (72). Accordingly, patients with T1DM have high rates of thyroid autoimmunity, especially if they are females (98) (99), and are at increased risk for Addison's disease (99), atrophic gastritis (100), pernicious anemia (98), celiac disease (101), and vitiligo (102).
Table 5.
Autoantibody Targets in Type 1 Diabetes
| glutamic acid decarboxylase 65 |
| Islet cells |
| Insulin |
| Zinc Transporter 8 |
Antigen Specific Cellular Immunity in Type1 Diabetes
Autoreactive T cells that develop in impending T1DM, localize to the pancreatic islets where they become a component part of the evolving insulitis lesions. Thus, circulating autoreactive T cells are relatively sparse in impending T1DM. Nevertheless, antigen specific T cells are identifiable through prolonged in-vitro cultures in the presence of purified or recombinant islet cell autoantigens such as GAD (103) (104) and IA-2 (105). In fact, autoreactivity to a large number of autoantigens have been reported in both human and murine diabetes (106). T cell proliferative responses to insulin and GAD65, and more generally to islet extracts, have been repeatedly reported in both patients with T1DM (107,108) and NOD mice. However, both in humans and NOD mice, reports of spontaneous proliferative responses have been difficult to reproduce and validate, probably because of the relative paucity of autoreactive T cells in peripheral blood samples, and the ready contamination of recombinant "test" antigens by lymphotoxin or lipopolysaccharide (LPS), that by itself, can produce proliferative responses even when present in trace amounts. Furthermore, significant T cell responses to insulin, proinsulin or GAD65 antigen were reported, in some normal controls as well as in T1DM patients (109-111). Numerous laboratories have reported T cell reactivity in diabetic patients against GAD65 and IA-2 and their peptides with variable results (105,107,112-117). However, in established diabetes, the loss of the majority of ß cell mass resulting in associated loss of GAD65 and other ß cell antigens, in turn leads to the inactivation of T cells due to the loss of the peptide antigens that were driving the response. Thus, antigenic/epitopic spreading is an undesirable phenomenon associated with progression in autoimmune diseases like T1DM to a clinically significant outcome.
Pathogenesis of Type 1 Diabetes
The availability of Biobreeding (BB) rats and nonobese diabetic (NOD) mice, the rodent models of T1DM, has greatly enhanced our understanding of the possible pathogenic mechanisms involved (Fig. 3). Recently, it has become possible to compare these findings with findings in human islets, obtained from post mortem specimens of the pancreas through the network of Pancreatic Organ Donors (nPOD) and from patients with recent onset DM via endoscopic pancreatic biopsy (DiViD study, Norway) (86,118,119). In addition, epidemiological studies aimed at the prediction and prevention of T1DM permit a picture of the natural history to emerge. The process of destruction of β-cells is chronic in nature, often beginning during infancy and continuing over the many months or years that follow. At the time of clinical diagnosis of T1DM, about +80% of the β- cells have been destroyed, the islets are infiltrated with chronic inflammatory mononuclear cells (insulitis), including CD8+ cytotoxic T cells. Once islet cell autoimmunity has begun, progression to islet cell destruction is quite variable, with some patients rapidly progressing to clinical diabetes, while others remain in a non-progressive state.

Figure 3.
The pathogenesis of islet cell destruction. Islet cell proteins are presented by antigen presenting cells (APCs) to naïve Th0 type CD4+ T cells in association with MHC class II molecules. Interleukin (IL)-12 is thus secreted by APCs that promotes the differentiation of Th0 cells to Th1 type cells. Th1 cells secrete IL-2 and IFN-γ that further stimulate CD8+ cytotoxic T cells or macrophages to release free radicals (super-oxides) or perforin/granzymes, leading to ß cell apoptosis or death. CD8+ cytotoxic T cells further mediate ß cell death by Fas mediated mechanisms. Interleukin (IL)-4, on the other hand, secreted mainly by natural killer T (NKT) cells drives Th0 cell to Th2 pathway leading to benign insulitis.
Diabetes risk and time to diabetes in relatives of patients directly correlates with the number of different autoantibodies present. The pathogenesis of T1DM has been extensively studied, but the exact mechanism involved in the initiation and progression of β-cell destruction is still unclear. The presentation of beta cell-specific autoantigens by antigen- presenting cells (APC) [macrophages or dendritic cells (DC)] to CD4+ helper T cells in association with MHC class II molecules is considered to be the first step in the initiation of the disease process. Macrophages secrete interleukin (IL)-12, stimulating CD4 + T cells to secrete interferon (IFN)-γ and IL-2. IFN-γ stimulates other resting macrophages to release other cytokines such as IL-1β, tumor necrosis factor (TNF-α) and free radicals, which are toxic to pancreatic β-cells. During this process, cytokines induce the migration of β-cell autoantigen specific CD8+ cytotoxic T cells. On recognizing specific autoantigen on ß cells in association with class I molecules, these CD8+ cytotoxic T cells cause ß cell damage by releasing perforin and granzyme and by Fas-mediated apoptosis of the beta cells. Continued destruction of beta cells eventually results in the clinical onset of diabetes.
Recently, these concepts derived from studies in the rodent models have been challenged as having the same pathologic process that occur in humans. Analysis of variations in histopathology observed from these organ donors provide mechanistic differences related to etiological agents and serve an important function in terms of identifying the heterogeneity of T1D (120). The findings are not always consistent with those of the rodent models. For example, the dense infiltration of islets by T-cells is evident in the pancreas of those who succumb to DKA at onset, but more chronic cases show a patchy distribution of destroyed and functioning islets containing beta cells with insulin suggesting a defect in secretion rather than synthesis. In the DiViD (Diabetes Virus Detection) study, expression of inflammatory markers, predominance of Class I antigens (rather than expression of Class 2 antigens) in islets, and actual viral isolations suggest a more acute process. Taken together, the studies suggest that T1DM may be a heterogeneous group of conditions in which auto-immunity may be a consequence or companion rather than the initiating mechanism. These findings begin to explain why prediction of developing T1DM in those from affected families considered at risk has become quite accurate, whereas prevention or reversal of DM by immune intervention or modulation has failed repeatedly (3,4,121).
The Inductive Event in Type 1 Diabetes
Various mechanisms have been proposed:
MOLECULAR MIMCRY
In antigenic molecular mimicry, cross-reactive immune responses occur due to significant structural homologies shared by molecules encoded by dissimilar genes.
The incidence of T1DM has increased over the last three to four decades in Europe, and the clinical disease exhibits preferential seasonal onset (122). These observations emphasize the role of environmental factors in the disease process. It has long been suggested that T1DM in humans is caused by viral infections (123-125). However, despite a vast increase in the information regarding the various genetic factors controlling the disease, little is known about the role of the putative environmental factors that might provide a more direct approach to therapy (8). Specifically, allegations that childhood vaccines could be causal have not been upheld by more extensive controlled studies.
The disease pathogenesis may involve multiple factors including the genetics of the host, strain of the virus, activation status of the autoreactive T cells, upregulation of pancreatic MHC class I antigens, molecular mimicry between viral and ß cell epitopes and direct islet cell destruction by viral cytolysis. Viruses, as one of the environmental factors affecting the induction of T1DM, may act as triggering agents of autoimmunity or as primary injurious agents, which directly damage pancreatic ß cells. Immune responses against a determinant shared by host cells and a virus could cause a tissue-specific immune response by generation of cytotoxic cross-reactive effector lymphocytes or antibodies that recognize self-proteins located on the target cells.
Monoclonal antibodies against viruses have been observed to be capable of cross-reacting with host determinants (126).
Several studies in humans also point to viruses as triggers of the disease (127). Coxsackie B4 virus and rubella virus have been linked with T1DM. In a few instances, Coxsackie B4 virus has even been directly isolated from pancreatic tissues of individuals with acute T1DM. Inoculation of this virus into mice, in one report, produced diabetes (128). The possibility that viruses might cause some cases of T1DM by infecting and destroying pancreatic ß-cells has received considerable attention. However, it is difficult to demonstrate in-vivo that viruses replicate in human ß-cells and/or produce diabetes in man. An in-vitro system was therefore developed to determine whether viruses are capable of destroying human β-cells in culture (129,130). By this method, it was clearly shown that several common human viruses, including mumps virus (131), Coxsackie B3 virus(132), Coxsackie B4 virus (128), reovirus type 3 (133), could infect human ß-cells. In addition, by radioimmunoassay, it was shown that the infection markedly decreased the insulin content of the ß-cells.
A strong correlation was found between the CMV genome in the immunocytes and the islet cell autoantibodies in the sera from diabetic patients (134). About 15% of newly diagnosed autoimmune T1DM patients have been reported to have persistent CMV infections.
Furthermore, it has been proposed that a molecular mimicry between protein 2C (p2C) of Coxsackie virus B4 and the autoantigen GAD65 may play a role in pathogenesis of T1DM. Kaufman et al (135) and Vreugdenhil et al (125), showed that the amino acid sequence of p2C shares a striking homology with a sequence in GAD65 (PEVKEK) and is highly conserved in Coxsackie virus B4 isolates as well as in different viruses of the subgroup of Coxsackie B-like viruses. These are the most prevalent enteroviruses and therefore the exposure to the mimicry motif should be a frequent event throughout the life. Furthermore, they suggested that molecular mimicry might be limited to the HLA-DR3 subpopulation of the T1D patients.
Although numerous sequence similarities between viral proteins and ß-cell autoantigens are plausible, the relationship between Coxsackie virus infection and GAD65 autoimmunity has received the most attention.
Glutamate Decarboxylase (GAD)
The finding by Kauffman et al (135), of a striking sequence homology of 18 amino acid peptide between human GAD65 and the Coxsackie virus p2-C protein, enhanced the evidence of a specific molecular mimicry model involving GAD. In addition, this specific region of GAD65 contains a T cell epitope involved in the GAD cellular autoimmunity in humans with immune mediated diseases (103) and this region is an early target of the cellular immunity in NOD mice (136,137). GAD catalyzes the formation of the inhibitory neurotransmitter γ-amino butyric acid (GABA) from glutamine (104). Two forms of GAD exist (GAD65 and GAD67). GAD65 is the predominant form within the human pancreatic islet cells, while GAD67 predominates in mouse islets. Within the islets, GAD is predominantly observed within the ß-cells, while its roles in the inhibition of somatostatin and glucagon secretion and in the regulation of proinsulin synthesis and insulin secretion, have also been suggested (138).
Another study further supports a link between Coxsackie virus and T1DM, associating IgM antibodies to Coxsackie B virus as a marker of recent exposure to the virus in newly diagnosed IMD patients and age/sex-matched controls (139). In that report, humoral immunity to Coxsackie virus and GAD appeared to cluster, even in people without diabetes. A series of overlapping synthetic GAD65 peptides were used to study the most reactive T cell determinants in individuals at increased risk for T1DM, i.e., autoantibody positive, first degree relatives of T1DM patients. Elevated in vitro T cell responses were observed to GAD65 peptides (amino acids 247-266 and 260-279) in newly diagnosed T1DM patients and autoantibody positive at- risk individuals (140). The sequence of this region of GAD65 (amino acids 250-273) is significantly similar to the p2-C protein of Coxsackie B virus (123). However, not all published reports have demonstrated a linkage between immunity to GAD and Coxsackie virus. For example, one study identified a non-Coxsackie-homologous region of GAD65 as a predominant cellular immune epitope while studying the polyclonal human T cell responses (115).
Insulinoma Antigen Two (IA-2)
Tyrosine phosphatase IA-2 is another molecular target of pancreatic islet autoimmunity in T1DM. In one recent study, the epitope spanning 805-820 amino acid elicited maximum T-cell responses in all at-risk relatives, out of a total of 68 overlapping, synthetic peptides encompassing the intracytoplasmic domain of IA-2 (141). This epitope was found to have 56% identity and 100% similarity over 9 amino acids with a sequence in VP7, a major immunogenic protein of human rotavirus. This dominant epitope also has 75-45% identity and 88-64% similarity over 8-14 amino acids to sequences in Dengue, cytomegalovirus, measles, hepatitis C and canine distemper viruses and the bacterium Haemophilus influenzae.
Furthermore, three other IA-2 epitope peptides have 71-100% similarity over 7-12 amino acid stretch to herpes, rhino-, hanta- and flavi-viruses. Two others have 80-82% similarity with dietary proteins of milk, wheat and bean proteins. These molecular mimicries could lead to triggering or exacerbation of ß-cell autoimmunity.
SUPERANTIGENS
Besides molecular mimicry, retroviral expression of superantigens (Sags) may be able to activate clonal expansion of autoreactive T cell clones. Superantigens have been implicated in the pathogenesis of the various autoimmune diseases (142,143). Originally described as minor-lymphocyte stimulating antigens, retroviral Sags expressed by B cells interact with the development of T helper cells of both Th1 and Th2 subtypes in mice. A study in patients with T1DM demonstrated that two thirds of IAA positive sera also reacted with p73 (144). Conrad et al (145) isolated a novel mouse mammary tumor virus-related human endogenous retrovirus (HERV), in patients suffering from acute onset T1DM. He termed them the HERV IDDMK1,2 22 subtype. They further showed that the N-terminal moiety of the envelope (env) gene encoded an MHC class II-dependent superantigen. He proposed that expression of this Sag, induced extra-pancreatically and by professional antigen-presenting cells, could lead to ß-cell destruction via the systemic activation of autoreactive T cells. He further reported the selective expansion of Vß7+ T cells in the islet cell infiltrates from two patients with recent onset IMD was associated with extensive junctional diversity of Vß7+ T cell clones. These investigators demonstrated that islet cell membrane preparations preferentially expanded Vß7+ T cells from non-diabetic peripheral blood mononuclear cells (146). However, other investigators were unable to confirm T1DM specificity of the IDDMK1,2 22, since it was equally recoverable as viremia from controls as well as patients (147). Furthermore, both patients and controls made antibodies to env proteins.
In order to establish molecular mimicry as a mechanism responsible for the autoimmune diseases it is important to identify the precise epitope that initiates the putative cross-reactive immune response. Additional complexity that has come to various animal studies is that of epitope spreading (148). An increasing array of autoantigens or autoantigenic peptides reactive with autoantibodies develop over time. Both intramolecular and intermolecular epitope spreading has been described in NOD mice (136,149). These studies demonstrated that T- cell responses in NOD mice expand in vivo against a defined group of islet cell antigens in an orderly sequential manner. These responses in the young NOD mice first show a strong reactivity to GAD enzyme and not to other islet cell antigens. Furthermore, the initial response to GAD is first limited to one region of the protein only. Gradually, this response spreads intramolecularly to involve other regions of the protein. Eventually, after the destructive islet cell inflammation (insulitis) as a result of autoimmunity to ß-cells, the T-cell responses spread intermolecularly to involve other islet cell proteins (e.g., heat shock protein 60, carboxypeptidase H and insulin) as well (150). This epitope spreading makes it difficult to predict which putative cross-reactions, if any, are important in terms of disease induction, and which do not give rise to autoimmune pathology, particularly in humans who are exposed to many infections.
Deficiencies in immunoregulation in Type 1 Diabetes
There is both evidence for and speculation about defective central and peripheral mechanisms of immunoregulation in the autoimmune form of T1DM. Deletion of autoreactive T cells in the thymus, is one mechanism for the induction of tolerance to self-antigens (central deletion). This may involve diminished expression of insulin in the thymus of susceptible individuals due to the presence of class I VNTR alleles 5' to the insulin gene as already discussed. Others have suggested that it is the ineffective antigenic binding of the T1DM-prone HLA-DQ or -DR that promotes islet cell autoimmunity, since this permits autoreactive T cells to escape thymic ablation and pass into circulation.
In addition to clonal T cell deletion and anergy in thymus, peripheral regulatory T (Treg) cells are essential for the down regulation of T cell responses to both foreign and self-antigens, and for the prevention of autoimmunity. Various studies have identified defects in the peripheral Treg cells in T1DM patients (151,152) as well as in NOD mice affecting both NKT cells (153,154) as well as CD4+CD25+ suppressor T cells (155). Since these Treg cells are not absent in either species, ways to stimulate them should be actively sought to provide novel therapies for the future. The possibility of future therapeutic use of Treg cells in human autoimmune diseases lies heavily on basic studies that are designed to elucidate the mechanisms of induction and function of these cells. Therapy with immunomodulatory compounds that specifically target endogenous pools of Treg cells can be envisioned (156). This approach requires a more detailed investigation into the intracellular and extracellular events that regulate the differentiation and expansion of these cells in-vivo.
Of great interest has been the emergence of immune mediated T1DM in patients treated with checkpoint inhibitors for various cancers (157). Unlocking the immune response via drugs that block the molecules programmed death (PD1) or its ligand, PDL1, as well as CTL4, may result in immunotoxicity with emergence of autoimmunity affecting various organs, including endocrine tissues such as the thyroid, adrenal and pancreas causing a form of T1DM (158). Indeed, autoimmunity has been called the “Achilles’ Heel” of immunotherapy, with increasing reports of its association with T1DM (159).
Environmental Factors in Type 1 Diabetes
Besides the familial predispositions, much evidence points to a major role of environmental factors in the disease pathogenesis. More than 60% of identical twins affected by T1DM are discordant for the disease and most of the non-diabetic twins lack islet cell autoantibodies. Over the past 3 decades, the disease frequency is on a steep rise in Western countries that cannot be explained by the accumulation of the susceptible genes. Africans, who dominate the tropics, and Chinese, both have low frequencies of the susceptible genes and low incidence rates of T1DM (75), except where there has been a high rate of Caucasian genetic admixture.
More persuasively, migrants from countries with low hygiene and low incidence rates of T1DM to countries with high hygiene and high incidence become as susceptible as the natives within a generation (160). Animals reared in sterile environments have early onsets and increased frequencies of diabetes while those infected with a variety of micro-organisms and parasites become protected (161-165). The hygiene hypothesis was proposed. A strong causal relationship between prevailing level of community hygiene, especially with respect to drinking water and the dramatic increase in the incidence of autoimmune diseases such as T1DM in the modern world, has been referred to as the hygiene hypothesis.
ROLE OF DIET
Despite persuasive epidemiological evidence for environmental factors that precipitate T1DM in genetically susceptible individuals, their identity remains elusive. This may be due to long period between exposure and the onset of hyperglycemia, the complex genetics of the disease, and the likely multiple insults of perhaps different derivation involved in the initiation of the insulitis and subsequent ß cell destruction. Dietary habits such as consumption of dairy products and early weaning of infants, and dietary toxins such as nitrates and nitrites have been associated with this autoimmune disease (166,167).
Close correlations between per capita consumption of unfermented milk proteins and the incidence of diabetes between countries(168-170) and within a country have been reported (171). The claimed negative association between diabetes incidence and a high frequency and long duration of breast-feeding is more controversial (166) and has not been confirmed by reports from Germany (172) and the United States. Several studies have found associations between the consumption of foods rich in nitrates (or nitrites), which is reduced to nitrite in the gut, and the occurrence of T1DM (173,174). The active species is believed to be N-Nitroso compounds that can be formed from the reaction of nitrite with amines (175). Most recently, the gut microbiome and its modulation by dietary factors, has been implicated in the causality of T1DM (176).
The incidence of T1DM varies worldwide according to dietary patterns. In-depth exploration of dietary risk factors during pregnancy and early neonatal life is warranted to confirm whether and to what extent diet cooperates with genetic susceptibility in the early onset of T1DM.
Screening Methods for Type 1 Diabetes
T1DM is by far the most common chronic metabolic disease of childhood and adolescence and its prevalence and incidence has been increasing worldwide (96). This increase of incidence is the highest among the children under 5 years of age (177). Prevention of T1DM would constitute a major advance in the lives of pre-diabetic individuals and significantly relieve a major current and predicted burden on both the individual and the health care system. Identifying individuals at risk developing the disease and the prevention of the disease progression are two important steps before the onset of disease. The presence of islet autoantibodies, as well as the genetic predisposition with specific HLA haplotypes are known risk factors associated with the development of diabetes. Most studies have been carried out on first-degree relatives of T1DM patients who have 15-fold increased risk of the developing diabetes in comparison to the general population. However, more than 90% of all patients developing T1DM do not have an affected family member. Therefore, it is crucial to establish a standardized screening method which will efficiently identify individuals at high risk in a general population. School children between 5-18 years of age were screened to evaluate the predictive value of autoantibodies over a period of 6-12 years (178). This study indicated that the risk of developing T1DM when ICA is detected in the absence of other autoantibodies is low, whereas with more than one autoantibody (either GAD65A, IAA, IA-2A or IA-2ßA) the risk of developing T1DM in a general population is high. Similar findings were also reported in other studies (179-181). These results support the value of multiple autoantibodies as good predictive markers for T1DM not only in first degree relatives but also in the general population. Consequently, the American Diabetes Association now considers the presence of 2 or more autoantibodies as form of early presymptomatic diabetes (182).
Prevention Trials in Type 1 Diabetes
The elucidation of the natural history of pre-diabetes has allowed for the characterization of those individuals at greatest risk for developing autoimmune T1DM, through the use of genetic, immunologic and metabolic markers. This predictive ability has become possible in both high- risk relatives and the general population as mentioned above. The subclinical autoimmune destruction of ß-cells in the pancreas may last from a few months to several years. This pre- diabetic period has allowed investigators to test prevention strategies, which mainly have focused in modulation of autoimmune process (183). A number of studies initiated with general immunosuppressive agents, such as cyclosporin-A, azathioprine and prednisone in patients with new clinical onset T1DM, positive results in that insulin free remission rates were increased and endogenous insulin (C-peptide) reserves were improved (121). However, despite continued immunotherapy with the attendant risks of renal damage and lymphomas at higher doses, relapses proved to be the rule and such treatments were abandoned. Cyclosporin given at a prediabetic phase of the disease delayed but did not prevent diabetes (184,185).
With the observation that nicotinamide prevents pancreatic ß cell destruction from streptozotocin by raising otherwise depleted levels of islet cell NAD as a result of superoxide induced DNA breaks and repair, the vitamin was subjected to a large European and Canadian trial called The European Nicotinamide Diabetes Intervention Trial (ENDIT). However, nicotinamide failed to prevent progression to diabetes (186). In addition, a study in Germany (DENIS) was completed without any effect of nicotinamide on prevention of T1DM.(187).More recent studies have used Anti CD21(Rituximab), Anti CD3, Anti CTLA-4, oral insulin,GAD65 peptides, and infusions of Treg cells with early encouraging results in preserving insulin secretion, but without durable effects (188). These results in humans were often based on animal studies in NOD mice (189-191). In stark contrast to these encouraging studies in NOD mice, where a variety of interventions induce long lasting remissions, none of the studies in humans has so far yielded long-lasting remissions in humans (183,188).
Table 6.
Prevention Trials (121)
| Study and Phase | Drug | Age | Eligibility | Ref |
|---|---|---|---|---|
| TRIGR | Cow’s milk hydrolysate | 0-7 days | First Degree relatives, High-risk HLA | (192) |
| BABY DIET | Gluten-free diet | Younger than 3 months | Relatives, high risk HLA DR, DQ | (193) |
| TrialNet NIP | Docosahexaenoic acid | >24 weeks gestation- newborn | Relatives, HLA DR3 or DR4 | (194) |
| TrialNet Teplizumab | Teplizumab | 8-45 years | At least 2 confirmed autoantibodies and abnormal glucose tolerance | (195,196) |
| DIAPREV-IT | GAD-alum | 4-18 years | Islet autoantibody positive | (197) |
| TrialNet Oral Insulin, Phase III | Human insulin | 1-45 years | Relatives, 2+islet antibodies including to insulin | (198) |
| INIT I/II, | Intranasal insulin | 4-30 years | Relatives, 2+islet antibodies, HLA not DR2, DQ6 | (199) |
| Pre-Point, Phase I/II | Human insulin | 1.5-7 years | First degree relatives, >50% risk of T1DM | (200) |
| FINDIA | Insulin-free whey- based formula | Infants | General population, high-risk HLA DQ | (201) |
| Teplizumab | Teplizumab | </=18 years of age | Relatives | (202) |
| Golimumab | Golimumab | 6 to 21 years | Newly diagnosed T1DM | (203) |
TYPE 2 DIABETES MELLITUS
As the US passed into the 21st century, the epidemic of obesity and T2DM continues unabated, affecting more younger adults and children than in the past. They will spend longer periods of their life with the disease. Perhaps in part under pressure of commercial interests, we as a nation have learned to eat too fast, too much, and the wrong foods. However, the problem of obesity and its consequences is pervasive globally, affecting developing as well as economically developed countries. For those with the energy conserving "thrifty" genes of insulin resistance syndrome (IRS), this excess of food and especially of the insulin provoking carbohydrates, leads to obesity, an IRS phenotype and T2DM. Nearly half of the new cases of diabetes in teens can be termed T2DM (204). Currently, in some US states where there are large numbers of ethnic groups prone to IRS and T2DM (Hispanics, American Indians, Asian Indians, African Americans), the number of children with T2DM is beginning to rival if not surpass the number with T1DM. It is estimated that 1 in 3 people born in the US in the year of 2000 will develop T2DM sometime in their lifetime (205).
The increased incidence of T2DM is attributed to the increase in obesity worldwide. Approximately 3700 youths are diagnosed with T2DM every year in the US (206) and it is estimated that the number of youth with T2DM will almost quadruple from 22,820 in 2010 to approximately 85,000 adolescents with T2DM by 2050 (10). Similar rates of increased in youths with T2DM are reported from the UK, India, China and Japan (10).
Pathophysiology of Type 2 Diabetes
T2DM is characterized by insulin resistance in peripheral tissues (muscle, fat, and liver) with progressive β cell failure, ,especially manifest with defective insulin secretion in response to a glucose stimulus, increased glucose production by the liver, and no markers of pancreatic autoimmunity (207). The progressive decline in β cell function is more rapid in youths at 20-30% decline per year versus 7-11% decline per year in adults, even with aggressive medical therapy.
Table 7.
Pathophysiologic Factors
| Obesity/Insulin resistance (IR) | See IRS |
| Intrauterine environment | Epidemiological studies have shown a strong association between poor intrauterine growth and the subsequent development of the Metabolic Syndrome. It was suggested that the effects of poor nutrition in early life impair the development of pancreas and resulting permanent changes in glucose- insulin metabolism (208). |
| Gestational diabetes | Studies in Pima Indian women showed significant increased risk of developing T2DM in offspring of women with diabetes during pregnancy compared to non-diabetic mothers (209). |
| Ethnicity | There is a significant increase risk in certain ethnic/race groups (205). |
| Gender and puberty | Puberty is a state of IR brought about by the increased secretion of GH during this process. There is a 30%-50% decrease in insulin sensitivity and compensatory increase in insulin secretion. Those that have an inherent defect in insulin secretion and inadequate response to the resistance develop DM. The mean age at diagnosis of T2DM in children is 13.5 years, corresponding to the time of peak adolescent growth and development. Girls are 1.5-3 times more likely than boys to develop T2D as children or adolescents (270). |
| Family History | Between 74-100% of children with T2DM have a first or second-degree relative with T2DM. The lifetime risk is 40% if one parent is affected and 70% if both parents are affected (210). |
| Genetics | Genome-wide studies led the discovery of single- nucleotide polymorphisms (SNPs) at several loci regulating insulin secretion. To date, more than 30 diabetes-related SNPS (diabetoSNPs) have been identified (211). Several genes have been found to be associated with T2D;
|
The natural history of progression to T2DM is that a person with IRS begins to decompensate, with a fall in the disposition index (the amount of insulin produced for the degree of insulin resistance). Subsequently levels of blood glucose rise after feeding; elevations in fasting blood glucose levels occur later. At this early stage, diet, exercise and insulin sensitizers are indicated.
INSULIN RESISTANCE SYNDROME (IRS)
This syndrome complex is centered upon genetic predispositions to insulin resistance and the hyperinsulinemia that results from it. This medical state is also named syndrome X and the metabolic syndrome, however the descriptive term insulin resistance syndrome (IRS) is the one increasingly used in the literature (207,212). In IRS, there are poorly understood genetic lesions that lead to insulin resistance from early life if not during embryogenesis. In many affected families, the disease occurrences suggest a dominant mode of transmission. In rare families, mutations affecting insulin receptors, or peroxisome proliferators-gamma (PPAR- gamma) expression may be the cause of it (213). IRS is the association of insulin and leptin resistance with obesity (typically with increased visceral fat), functional adrenal hyper-androgenism, functional ovarian hyperandrogenism, hypersecretion of pituitary LH, dyslipidemia, hypertension, and features of hyperinsulinemia such as late reactive hypoglycemia and acanthosis nigricans. When the compensation by increased insulin secretion fails, glucose intolerance and T2DM result.
Natural History of Insulin Resistance Syndrome
Several studies indicate that many children and adults with T2DM were born small for gestational age. This suggests that the insulin resistant state existed in-utero since it is insulin rather than pituitary growth hormone that is the principal growth-promoting hormone of the unborn child, and decreased insulin action might be anticipated to impair embryonic growth. After birth, premature pubarche resulting from excessive adrenal androgens such as dihydroepiandrosterone (DHEA) may occur, even before obesity has appeared. Thus, it has been proposed by some that obesity may be the result of insulin resistance, and not its cause. Excessive DHEA may be seen best after ACTH injection leading to a clinical suspicion that the 3ß hydroxysteroid dehydrogenase enzyme is underactive. Obesity can begin from infancy but often dates from about 8 years of age when physiological pubarche occurs. Early onset obesity raises the possibility of a genetic satiety causation such as the Prader-Willi Syndrome or deficiency of MC4R. Acanthosis nigricans resulting from increased keratinocytes in certain areas of skin is thought to result from insulin stimulation of insulin-like growth factor 1 (IGF-1) receptors and often manifests during puberty Menarche may be delayed in age at onset or menses may be missed after menarche, or else there can be dysfunctional bleeding resulting from anovulatory cycles.
Hirsutism often becomes bothersome during adolescence, as may male pattern hair thinning, persistent acne and development of polycystic ovaries. An increase in very low-density lipoprotein (VLDL) secretion by the liver is observed with increasing age, associated with diminished, atherogenesis protective, high density lipoprotein cholesterol (HDL-C), a dyslipidemic profile that promotes early and progressive onset of atherosclerosis, predisposing to coronary heart disease (CHD), stroke, and peripheral vascular diseases in later life. The latter problems are compounded by the appearance of hypertension and type-2 diabetes. The glucose intolerance that precedes type-2 diabetes often first involves post-prandial glucose levels or the two-hour time point of the OGTT as discussed above, but later induces a rise in fasting glucose (impaired fasting glucose) levels as well. The mechanism is thought to be ß cell exhaustion or more likely a glucosamine and lipid mediated islet cell toxicity. Once this stage is reached, damage to the islets can become irreversible, resulting in the dual problems of insulin resistance and insulinopenia, both of which need to be addressed in therapeutic strategies. In children and adolescents, the progression of impaired insulin secretion and its complications including the appearance of albuminuria, exhibits a faster tempo than that of adults presenting later in life. Hence, these adolescents may more rapidly progress to requiring insulin therapy.
Table 8.
| Clinical Features | |
|---|---|
| Infancy | Family history of obesity and T2DM, SGA, LGA Gestational Diabetes |
| Childhood/Adolescence | Acanthosis nigricans Premature adrenarche, Obesity, Pseudoacromegaly, Striae, Skin tags, Amenorrhea |
| Adulthood | Tall Stature, Pseudoacromegaly Fatty liver, Focal glomerulosclerosis Hirsutism, Ovarian hyperandrogenism, PCOS Endothelial dysfunction, Atherosclerosis, Increased carotid wall thickness, Stroke CHD Glucose intolerance, T2DM |
Table 9.
Laboratory Features of IRS
| ↓IGFBP-1, ↓SHBG, ↑free testosterone ↓CBG, ↑free cortisol ↑VLDL, ↑TG, ↓HDL, ↑ small dense LDL Increased PAI-1, CRP, fibrinogen Adhesion molecules and uric acid Decrease first phase insulin response Increased decompensated insulin resistance Postprandial hyperglycemia Fasting hyperglycemia Diabetes |
Underlying Mechanisms of Insulin Resistance
OBESITY
Affected patients commonly show polyphagia, and may have voracious appetites that are characteristically resistant to dietary advice. When leptin deficiency was discovered in Ob/Ob mice and leptin receptor deficiency discovered in Db/Db mice, the adipocyte became to be appreciated as an endocrine cell rather than one that was an inert repository of triglycerides. However, the promise of a breakthrough in the understanding of human obesity was quickly dissipated when such lesions proved to be rare in humans. Obese patients with their greater degrees of adiposity also have the highest levels of leptin as expected, however these high levels do not reduce the appetites of IRS patients (215). Thus, such patients are also leptin resistant. Early trials of leptin therapy have not affected weight loss. However, patients with lipodystrophy who have leptin deficiency develop insulin resistance, hyper-insulinemia, dyslipidemia and T2DM, all of which respond dramatically to leptin given as therapy (216,217). Deficiencies in other appetite suppressing hormones such as resistin have more recently been implicated but not yet shown to have therapeutic relevance. Hyperinsulinemia itself is a compounding variable, in that excessive carbohydrate containing diets stimulate the highest levels of insulin and the greatest degrees of adiposity. Therapies such as metformin that improve insulin sensitivity when combined with a diet restricted in low amounts of simple carbohydrates and exercise, can dramatically lower weight in children with IRS when they adhere to therapeutic guidelines. However, failure to adhere to instructions is a common problem in adolescents (218,219).
HYPERANDROGENISM
It is uncertain as to the degree to which the pituitary abnormality of increased LH secretion leads to the androgenic excess or vice versa. Probably, both are responses to the insulin resistance and hyperinsulinemia of IRS by mechanisms that have yet to be clearly understood. Androgens of ovarian origins usually predominate over those of the adrenal gland, albeit both are often found to be elevated. Sex hormone binding globulins in the circulation are often low, resulting in increased free androgens with their increased bio-availability (220). This is often seen with testosterone, which can be raised or normal in hirsute girls whereas increased free testosterone levels are common.
Interestingly, we hold that there is a clinical overlap between Cushing's syndrome and IRS (221). Both tend to have visceral (central) obesity and striae suggestive of glucocorticoid excess. However, whereas the patient with Cushing's syndrome has high levels of serum cortisol, the patient with IRS has low normal levels, albeit both have increased levels of urinary free cortisol. Again, the explanation may lie in the low levels of corticosteroid binding globulins found in IRS where circulating cortisol is disproportionately free. Some investigators have suggested that there is an impaired conversion of cortisol to the metabolically inactive cortisone in IRS. Further, the child with Cushing's syndrome is invariably growth retarded in contrast to the child with IRS whose linear growth tends to be excessive. In IRS and obesity, the GH levels during stimulation tests are suppressed implying a diagnosis of GH deficiency which likely is not the case as these children tend to be tall. IGFBP levels in serum are depressed, resulting in an excessive free IGF-1 level, albeit the total IGF-1 concentration is usually normal. The pseudo-acromegaly observed in severely affected children with IRS may be occurring via this mechanism. In addition, high concentrations of insulin interact with the IGF-I receptor, thereby promoting growth (222).
ACANTHOSIS NIGRICANS
Stimulation of the IGF-1 receptors of skin keratinocytes by high levels of circulating insulin is thought to explain their hyperplasia and excessive laying down of keratin in the skin of the neck, axillae, elbows and knees, skin creases and indeed most areas of skin (223). In addition, excessive free IGF-1 may have the same effect, albeit the greater the degree of insulin resistance, the higher the insulin levels, the more striking the acanthosis nigricans. Increased bioavailability of IGF-1 (high IGF-1 and low IGFBP-1) are directly correlated with the severity of acanthosis nigricans
GLUCOSE INTOLERANCE AND T2DM
Children and young adults affected by IRS are often hyperinsulinemic. In such persons, stimulation of insulin secretion by carbohydrates alone or with protein can induce an excessive but delayed rise in insulin secretion, reflected in an early excessive rise in glucose, followed by an excessive fall in glucose levels 3-5 hours afterwards, of sufficient severity to provoke symptoms of hypoglycemia. As the ability to secrete insulin declines, impaired glucose intolerance appears first. Later in the evolution of T2DM, the 2-hour criteria for diabetes during OGTT become apparent, followed later by impaired fasting hyperglycemia and finally by fasting hyperglycemia that meets the criteria for the diagnosis of diabetes. An HbA1c level can be used to screen diabetes as recommended by the American Diabetes Association.
Table 10.
Criteria for Increased Risk of Diabetes (1)
| Fasting plasma glucose | 100 – 125 mg/dl |
| 2-hour plasma glucose after OGTT | 140 – 199 mg/dl |
| HbA1C | 5.7 – 6.4% |
NON-ALCOHOLIC STEATOHEPATITIS (NASH)
It is also known as fatty liver or hepatic steatosis. The incidence of fatty liver among obese children was 2.6% in one study (224), and hyperinsulinemia was found to be the major contributor for its’ development (225). A number of factors may play a role in the development of fatty liver including, induction of cytochrome P4502E1 during obesity, which is capable of generating free radicals, while the high level of dietary intake of polyunsaturated fatty acids or low intake of nutritional antioxidants contributes to the oxidative stress. Fatty liver alone appears to be a relatively benign disease, and can be reversible. However, it may progress over years to hepatic cirrhosis, liver failure, or hepatocellular carcinoma. The onset of disease is usually insidious. Laboratory evaluation indicates mild to moderate elevation of serum aminotransferases in most children and serum alanine aminotransferase (ALT) levels had been shown a useful screening for fatty liver in obese children (226). The ratio of aspartate aminotransferase (AST) to ALT is usually less than 1, but this ratio increases as fibrosis advances. Serum aminotransferases, alkaline phosphatase and gamma glutamyl transferase (GGT) levels are proposed surrogate markers of fatty liver (227,228).
RENAL INVOLVEMENT
A form of focal glomerulosclerosis (often with IgA deposition) appears to be associated with IRS, leading to microalbuminuria. Hypertension becomes increasingly common through adolescence and beyond. The mechanisms responsible have not been elucidated.
INFLAMMATION
IRS and T2DM have increased markers of inflammation. This takes the form of increased levels of C-reactive protein, raised erythrocyte sedimentation rates (ESR) and increased cytokine (TNF-α) levels. Obese patients also have abnormalities of thyroid function suggestive of primary thyroid deficiency with modestly elevated TSH but normal or slightly elevated fT4 and fT3.These abnormalities resolve with weight loss and have therefore been interpreted as representing an adaptive response to obesity i.e., by raising TSH and free T3, caloric expenditure would increase (229-231). Obese patients are thus often unnecessarily treated for hypothyroidism they do not have. They may however develop true hypothyroidism on the basis of associated Hashimoto's disease.
ATYPICAL DIABETES
Genetic Defects of ß-cell Function (Monogenic Diabetes)
Monogenic forms of diabetes are characterized by impaired secretion of insulin from pancreatic β cells caused by a single gene mutation. These forms comprise a genetically heterogenous group of diabetes including, maturity onset diabetes of the young (MODY), permanent or transient neonatal diabetes (NDM), and mitochondrial diabetes. MODY is the most common form of monogenic diabetes, with autosomal dominant transmission of a gene encoding a primary defect in insulin secretion (232-235).
Approximately 1 to 2% of diabetes in Europe is MODY (236). The clinical characteristics of these patients are heterogeneous, and not reliable in predicting the underlying pathogenesis (237,238). It is often misdiagnosed as T1DM or T2DM. Several genetic abnormalities have been found that account for the disorder. Some members of an affected family may have the genetic defect but not develop the diabetes phenotype. Whether this is due to modifying genes or environmental factors is unclear. MODY differs from the classical immunological T1DM in several ways. With MODY, a dominant family history of diabetes (if known) is always present. However, de novo mutations can occur. Hyperglycemia is mostly mild with a minimal tendency to ketosis before the age of 25 years, the insulin secretion in response to oral (OGTT) or intravenous (IVGTT) glucose administration is modestly decreased, and evidence of islet cell autoimmunity is absent. It is estimated that more than 80% of patients with monogenic diabetes are either not diagnosed or are misclassified as type 1 or type 2 DM (239).
The underlying genetic defects of the many MODY subtypes have been identified, as indicated below (Table 11). To date, fourteen genetic forms of MODY are recognized. MODY resulting from defects in the glucokinase gene (GCK) and hepatocyte nuclear factor-1-alpha (HNF-1α) are the most common types seen during childhood (MODY-2) and post puberty (MODY-3), respectively. MODY Types 2 and 3 together constitute 80% of all cases of MODY syndromes.
MODY 2 is the most common form of MODY with a prevalence of about 1:1000 people. It is caused by a dominant heterozygous inactivating mutation in glucokinase, the enzyme that phosphorylates glucose to permit its oxidation to ATP and hence insulin release. Insulin is released but at higher glucose concentration-the curve is right shifted but otherwise normal. Thus, fasting glucose is in the range of ~95-110 mg/dl and may remain above 140 mg/dl at 2 hours post prandial but returns to normal thereafter. HbA1c is in the range of 5.8-7.6% and generally remains in the low- mid 6% range. Patients are rarely symptomatic and may be discovered by chance when a blood glucose is obtained. Treatment is not necessary except during pregnancy in some cases; there is a very low prevalence of micro-macrovascular disease even after almost 50 years of follow-up. Young women are often discovered to have mid hyperglycemia when tested during pregnancy and erroneously labeled as having gestational diabetes. The non-affected fetus of an affected Mother may have some macrosomia in utero-the result of extra insulin secretion by the fetus in response to the maternal hyperglycemia (240).
MODY3 is the next most common form of MODY caused by a heterozygous mutation in HNF-1α, necessary for normal insulin secretion. Onset is usually in the teen years and glucose is in the mid-200s with mild to moderate symptoms. Patients may respond to sulfonylurea drugs initially, but later may go on to insulin dependence and more severe hyperglycemia. As with other MODY forms, a family history of diabetes is often obtained, with a diagnosis of T2DM common for older patients and T1DM in younger patients. Confirmation of the diagnosis by molecular testing is essential for recommending treatment and family counseling (241).
Defects in four pancreatic ß cell-specific transcription factor genes, HNF-1β (MODY5), HNF-4α (MODY1), pancreatic and duodenal homeobox 1 gene (PDX1) [previously termed insulin promoter factor-1 (IPF-1)] (MODY4) and neurogenic differentiation 1 gene (NeuroD1) and BETA2 (MODY6) are responsible for others. In contrast to MODY-2, patients with heterozygous mutations in the HNF1A, HNF4A, or HNF1B and more rarely in PDX1 or NEUROD1 have progressive deterioration in glucose tolerance and are at risk for developing complications of diabetes (242).
More recently, mutations in the tumor suppressor protein KLF-11 (MODY7), the carboxyl ester lipase CEL (MODY8), the transcription factor, paired box gene 4, PAX-4 (MODY9), the insulin gene, INS (MODY10), and tyrosine kinase, B-lymphocyte specific gene, BLK (MODY11) have been described. MODY 12 and MODY 13 are due to mutations in the ABCC8 and KCNJ11 genes, respectively. Mutations in these 2 genes also have been reported in neonatal diabetes. They are very rare and represent fewer than 1% of all MODY cases.
Table 11.
Classification of MODY
| MODY Type | Gene Gene Loci | Incidence | Age at Diagnosis | Primary Defect | Associated Features | Severity of Diabetes | Ref |
|---|---|---|---|---|---|---|---|
| 1 | HNF-4α 20q | Rare | Postpubertal | Transcription gene defects in ß-cells lead to impaired metabolic signaling of insulin secretion. | - | Severe | (242) |
| 2 | Glucokinase 7p | 10-60% | Childhood | impairment of ß-cells sensitivity to glucose and; defect in hepatic glycogenesis | Reduced birth weight | Mild | (243) |
| 3 | HNF-1α 12q | 20-60% | Postpubertal | Similar to MODY1 | Renal glucosuria | Severe | (242-246) |
| 4 | PDX1 (IPF-1)13q | Rare | Early adulthood | Defects in transcription factors during embryogenesis lead to abnormal ß-cell development and function | - | Mild | (247) |
| 5 | HNF-1β 17cen- q21.3 | Unknown | Postpubertal | Similar to MODY 1 and 3 | Glomerulocystic kidney disease, female genital malformations, Hyperuricemia, abnormal liver function tests | Mild | (248) |
| 6 | NeuroD1/BETA2 2q32 | Rare | Early adulthood | Defect in this gene causes abnormal development of ß cell and function | - | Unknown | (249) |
| 7 | KLF11 2p25 | Very Rare | Early adulthood | Reduced glucose sensitivity of the beta cell | Phenotype similar to T2D | Unknown | (250) |
| 8 | CEL 9q34 | Very Rare | <20 years | Impaired endocrine and exocrine pancreatic function | Exocrine pancreatic dysfunction | Unknown | (251) |
| 9 | PAX4 7q32 | Very Rare | <20 years | Impaired gene transcription in pancreatic beta cells on apoptosis and proliferation | - | DKA is possible | (252,253) |
| 10 | INS 11p15.5 | Very Rare | <20 years | Defect in this gene may result the loss of beta cell mass through apoptosis | - | Unknown | (254) |
| 11 | BLK 8p23 | Very Rare | <20 years | decreases insulin synthesis and secretion in response to glucose by up- regulating transcription factors | Higher incidence in obese individuals | Unknown | (255) |
| 12 | ABCC8 11p15.1 | < 1% | <35 years | Inactivating mutations cause impaired secretion mild mode | (255) | ||
| 13 | KCNJ11 11p15.1 | <1% | |||||
| 14 | APPL1 3p14.3 | <1% | adapter protein, phosphotyrosine interacting with pH domain and leucine zipper | (256,257) |
Neonatal Diabetes
Neonatal diabetes is a rare disorder with an incidence of 1:100,000-1:200,000 live births (232,258). It presents in first 6 months of life and its’ severity depends on the underlying mutation in that it is either transient or permanent. Almost 50% of cases with neonatal diabetes are permanent (PND) while the remainder are “transient” (TNDM) in that they remit, but may reappear and become apparent later in life or at times of stress. Heterozygous activating mutations in KCNJ11 and ABCC8 —which encode the Kir6.2 and SUR1 subunits, respectively, of the ATP-sensitive potassium channel, are the most common causes of PND. Missense mutations in the INS gene are also identified in patients with PND and they may have an autosomal dominant or recessive inheritance pattern (232,254,258). Genetic diagnosis is important since the KCNJ11 and ABCC8 mutations respond to treatment by sulfonylureas, possibly without need for additional insulin therapy because these drugs can close the β cell potassium channel by an ATP-independent route (259). It is increasingly apparent that the same mutations can become manifest for the first time well beyond infancy and diagnosed as T2DM or rarely T1DM. Severe mutations in the KATP genes, especially KCNJ11 also may present with a neurological component in a syndrome known as DEND (Developmental delay, Epilepsy, Neonatal Diabetes); early diagnosis and treatment with sulfonylurea drugs is reported to ameliorate the neurological manifestations as the KATP channels are expressed in the brain. The major form of transient neonatal diabetes results from anomalies of the imprinted region on chromosome 6q24,but mutations in KCNJ11 or ABCC8 can also cause TNDM (232). Various rare forms of syndromic disease which include NDM are described; early diagnosis may diminish or delay the hitherto described natural history and consequences (258).
Mitochondrial Diabetes
Point mutations in mitochondrial m.3243A→G cause another form of diabetes with an insulin secretory defect that is commonly associated with neuro-sensory hearing impairment and a strict maternal mode of inheritance (260). In addition, genetic abnormalities that result in the inability to convert pro-insulin to insulin (261), or the production of mutant insulin molecules (262), are other examples of specific genetic defects in ß cell function which are rare causes of diabetes.
Chronic Illnesses
Hemochromatosis is a progressively more common recognized cause of diabetes with aging, and does not present in a pediatric age group. However repeated blood transfusions for conditions such as thalassemia major can lead to diabetes associated with hemosiderosis.
Many patients with cystic fibrosis develop a form of T1DM often during their teenage years which may require insulin replacement and is labeled “cystic fibrosis related diabetes (CFRD)” (263). Most CF patients now live long enough for this to have become a more common problem with impact on overall well-being and severity of symptoms ascribed to CF and partially responsive to insulin therapy. DKA is rare in CFRD, perhaps because of the concurrent effects on the α-cell secreting glucagon as well as the β-cell secreting insulin. Patients with Gitelman’s syndrome develop diabetes which resolves when they are adequately replaced with magnesium, excessively lost through the kidneys in this syndrome. Gitelman syndrome is a recessively inherited genetic entity, but the presentation of DM is usually not until later midlife (264).
Genetic Defects in Insulin Action
There are a series of rare genetic abnormalities in the insulin receptor, or in the signal transduction events which follow insulin docking to its receptor resulting in diabetes. The recessive DNA breakage disease (Bloom’s syndrome) is associated with mild diabetes due to severe insulin resistance, with very high levels of circulating insulin and insulin like growth factor one (IGF-1). Progeria and lipodystrophy are other such causes (232). In the latter case, the absolute deficiency of leptin leads to uncontrolled lipolysis resulting in severe insulin resistance, which is partially reversible by leptin administration (232)/
Endocrinopathies Associated with Hyperglycemia
Several hormones, such as epinephrine, glucagon, cortisol, and growth hormone, antagonize the action of insulin. Whereas release of these hormones constitutes the protective counter regulatory response to hypoglycemia, primary over secretion of these hormones can result in glucose intolerance or overt diabetes.
- Cushing's syndrome, due to pituitary and ACTH secreting adenomas or adrenal hyperplastic disease or to exogenous glucocorticoid administration, can lead to diabetes (265). Steroid-induced diabetes is most often seen when there is pre- existing insulin resistance or a defect in insulin synthesis/secretion unmasked by the inability to increase insulin secretion to overcome the resistance to its actions induced by glucocorticoids.
- Acromegaly is associated with overt diabetes in 10 to 15% of cases, and impaired glucose tolerance in a further 50% (266,267). In acromegaly, there is marked insulin resistance and hyperinsulinemic responses; DM occurs only when the hyperinsulinemic response cannot match the requirement to overcome the degree of resistance.
- Pheochromocytomas are associated with both inhibition of insulin secretion and an increase in hepatic glucose output (268). These changes lead to impaired glucose tolerance, the severity of which is directly related to the magnitude of catecholamine production (269). When seen in children, these are usually a component of the Von Hippel-Lindau syndrome, MEN2, and NF1.
- Glucagon-secreting tumors (glucagonoma) are associated with an unusual constellation of clinical features, including skin rash, weight loss, anemia, and thromboembolic problems. Approximately 80% of these patients have either impaired glucose tolerance or diabetes (270).
- Somatostatin-secreting tumors (somatostatinomas) are typically associated with the triad of diabetes mellitus, cholelithiasis, and diarrhea with steatorrhea (271).
- Although thyroxine is not a counter regulatory hormone, hyperthyroidism can interfere with glucose metabolism. It is associated with both increased sensitivity of pancreatic ß cells to glucose, resulting in increased insulin secretion, and antagonism to the peripheral action of insulin. The latter effect usually predominates, leading to impaired glucose tolerance in some untreated patients (272).
Drug- or Chemical-induced Diabetes
A large number of drugs can impair glucose tolerance; they may act by decreasing insulin secretion, increasing hepatic glucose production, and/or by causing resistance to the action of insulin (273). Included in this list are several classes of antihypertensive drugs, such as beta blockers (274), protease inhibitors used for the treatment of HIV infection (275), and tacrolimus and cyclosporine used primarily to prevent transplant rejection (276,277). Drugs of the serotonin re-uptake inhibitor (SSRIs) class can lead to obesity, impaired glucose intolerance and T2DM, especially if individuals were already insulin resistant before they started such medications.
There is a common association between obesity, insulin resistance, hypertension, and dyslipidemia, which has been called syndrome X or the metabolic syndrome (207,212,278,279). The administration of a thiazide diuretic or a ß-blocker to such patients can exacerbate the insulin resistance and may bring on hyperglycemia (274). In comparison, angiotensin-converting enzyme (ACE) inhibitors and alpha-adrenergic antagonists (such as doxazosin) may improve insulin sensitivity. Because the former also protect against renal disease, they are the drugs of choice for diabetic patients with hypertension.
Viral Infections
Certain viruses e.g., Coxsackie B4, have been implicated to cause diabetes, either through direct ß cell destruction or possibly by inducing autoimmune damage. The direct proof of this however remains tenuous. Chronic hepatitis C virus infection is associated with an increased incidence of diabetes, but it remains uncertain as yet if there is a cause-and-effect relationship.
Uncommon Forms of Immune-Mediated Diabetes
Several uncommon forms of immune-mediated diabetes have been identified.
- The stiff-man syndrome is an autoimmune disorder of the central nervous system, which is characterized by progressive muscle stiffness, rigidity, and spasms involving the axial muscles, with impairment of ambulation (280). Patients characteristically have high titers of glutamic acid decarboxylase (GAD65) autoantibodies and diabetes occurs in at least one-third of cases. Graves’ disease is also common in the syndrome. Presentation is usually in early adulthood.
- Anti-insulin receptor antibodies can bind to insulin receptors and either act as an agonist, leading to hypoglycemia, or block the binding of insulin and cause diabetes (281). This so-called type B insulin resistance is more common in females who show other signs of autoimmunity including systemic lupus erythematosus (SLE). However one study found that almost 10% of young patients with insulin resistance in the absence of autoimmune stigmata were also positive for insulin receptor autoantibodies (282).
REFERENCES
- 1.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15–S33. [PubMed: 33298413]
- 2.
- American Diabetes Association. Standards of Medical Care in Diabetes-2021 Abridged for Primary Care Providers. Clin Diabetes. 2021;39:14–43. [PMC free article: PMC7839613] [PubMed: 33551551]
- 3.
- Soleimanpour SA, Stoffers DA. The pancreatic beta cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab. 2013;24:324–331. [PMC free article: PMC3908840] [PubMed: 23647931]
- 4.
- Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, Pietropaolo M, Arvan PR, Von Herrath M, Markel DS, Rhodes CJ. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60:1370–1379. [PMC free article: PMC3292309] [PubMed: 21525508]
- 5.
- Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. [PubMed: 18502302]
- 6.
- Moltchanova EV, Schreier N, Lammi N, Karvonen M. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med. 2009;26:673–678. [PubMed: 19573115]
- 7.
- Diabetes Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990- 1999. Diabet Med. 2006;23:857–866. [PubMed: 16911623]
- 8.
- Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L. Study SfDiY. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376:1419–1429. [PMC free article: PMC5592722] [PubMed: 28402773]
- 9.
- Patterson CC, Gyurus E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C, Bingley PJ, Schoenle E, Jarosz-Chobot P, Urbonaite B, Rothe U, Krzisnik C, Ionescu-Tirgoviste C, Weets I, Kocova M, Stipancic G, Samardzic M, de Beaufort CE, Green A, Dahlquist GG, Soltesz G. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142–2147. [PubMed: 22638547]
- 10.
- Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, Rodriguez BL, Standiford D. Group SfDiYS. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35:2515–2520. [PMC free article: PMC3507562] [PubMed: 23173134]
- 11.
- Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, Gale EA. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364:1699–1700. [PubMed: 15530631]
- 12.
- VanBuecken D, Lord S, Greenbaum CJ. Changing the Course of Disease in Type 1 Diabetes. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth (MA)2000.
- 13.
- Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. [PMC free article: PMC5081106] [PubMed: 27061677]
- 14.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40:S11–S24. [PubMed: 27979889]
- 15.
- Standards of Medical Care in Diabetes-2017. Summary of Revisions. Diabetes Care. 2017;40:S4–S5. [PubMed: 27979887]
- 16.
- Ostergaard JA, Laugesen E, Leslie RD. Should There be Concern About Autoimmune Diabetes in Adults? Current Evidence and Controversies. Curr Diab Rep. 2016;16:82. [PubMed: 27457237]
- 17.
- Greenbaum CJ, Speake C, Krischer J, Buckner J, Gottlieb PA, Schatz DA, Herold KC, Atkinson MA. Strength in Numbers: Opportunities for Enhancing the Development of Effective Treatments for Type 1 Diabetes-The TrialNet Experience. Diabetes. 2018;67:1216–1225. [PMC free article: PMC6014559] [PubMed: 29769238]
- 18.
- Ganda OP, Srikanta S, Brink SJ, Morris MA, Gleason RE, Soeldner JS, Eisenbarth GS. Differential sensitivity to beta-cell secretagogues in "early," type I diabetes mellitus. Diabetes. 1984;33:516–521. [PubMed: 6373458]
- 19.
- Cantor AB, Krischer JP, Cuthbertson DD, Schatz DA, Riley WJ, Malone J, Schwartz S, Quattrin T, Maclaren NK. Age and family relationship accentuate the risk of insulin-dependent diabetes mellitus (IDDM) in relatives of patients with IDDM. J Clin Endocrinol Metab. 1995;80:3739–3743. [PubMed: 8530627]
- 20.
- Rewers M, Norris JM, Eisenbarth GS, Erlich HA, Beaty B, Klingensmith G, Hoffman M, Yu L, Bugawan TL, Blair A, Hamman RF, Groshek M, McDuffie RS Jr. Beta-cell autoantibodies in infants and toddlers without IDDM relatives: diabetes autoimmunity study in the young (DAISY). J Autoimmun. 1996;9:405–410. [PubMed: 8816978]
- 21.
- Maclaren N, Lan M, Coutant R, Schatz D, Silverstein J, Muir A, Clare-Salzer M, She JX, Malone J, Crockett S, Schwartz S, Quattrin T, DeSilva M, Vander Vegt P, Notkins A, Krischer J. Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2beta predict immune-mediated (Type 1) diabetes in relatives. J Autoimmun. 1999;12:279–287. [PubMed: 10330299]
- 22.
- Randle PJ, Kerbey AL, Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988;4:623–638. [PubMed: 3069395]
- 23.
- Exton JH. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev. 1987;3:163–183. [PubMed: 3032541]
- 24.
- El-Maghrabi MR, Claus TH, McGrane MM, Pilkis SJ. Influence of phosphorylation on the interaction of effectors with rat liver pyruvate kinase. J Biol Chem. 1982;257:233–240. [PubMed: 6273426]
- 25.
- Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. [PubMed: 1562196]
- 26.
- Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153:1039–1048. [PMC free article: PMC3281526] [PubMed: 22166985]
- 27.
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. [PubMed: 4581053]
- 28.
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. [PMC free article: PMC3248306] [PubMed: 22214853]
- 29.
- Kimball SR, Jefferson LS. Cellular mechanisms involved in the action of insulin on protein synthesis. Diabetes Metab Rev. 1988;4:773–787. [PubMed: 3069402]
- 30.
- Kimball SR, Jefferson LS. Regulation of initiation of protein synthesis by insulin in skeletal muscle. Acta Diabetol. 1991;28:134–139. [PubMed: 1777648]
- 31.
- Cahill GF Jr. Starvation in man. Clin Endocrinol Metab. 1976;5:397–415. [PubMed: 182420]
- 32.
- McGarry JD. Lilly Lecture 1978. New perspectives in the regulation of ketogenesis. Diabetes. 1979;28:517–523. [PubMed: 437381]
- 33.
- Wolfsdorf J, Glaser N, Sperling MA. American Diabetes A. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–1159. [PubMed: 16644656]
- 34.
- Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med. 1983;309:159–169. [PubMed: 6408476]
- 35.
- Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF Jr. Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574–583. [PMC free article: PMC535723] [PubMed: 5773093]
- 36.
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. [PMC free article: PMC292907] [PubMed: 6061736]
- 37.
- Ashby P, Robinson DS. Effects of insulin, glucocorticoids and adrenaline on the activity of rat adipose-tissue lipoprotein lipids. Biochem J. 1980;188:185–192. [PMC free article: PMC1162554] [PubMed: 6996675]
- 38.
- Parkin SM, Walker K, Ashby P, Robinson DS. Effects of glucose and insulin on the activation of lipoprotin lipase and on protein-synthesis in rat adipose tissue. Biochem J. 1980;188:193–199. [PMC free article: PMC1162555] [PubMed: 6996676]
- 39.
- Tavangar K, Murata Y, Pedersen ME, Goers JF, Hoffman AR, Kraemer FB. Regulation of lipoprotein lipase in the diabetic rat. J Clin Invest. 1992;90:1672–1678. [PMC free article: PMC443223] [PubMed: 1430198]
- 40.
- Todd JA. Genetics of type 1 diabetes. Pathol Biol (Paris). 1997;45:219–227. [PubMed: 9296067]
- 41.
- She JX. Susceptibility to type I diabetes: HLA-DQ and DR revisited. Immunol Today. 1996;17:323–329. [PubMed: 8763818]
- 42.
- Noble JA, Cavalli AS, Erlich HA. DPB1*5901a: a novel HLA-DPB1 allele from a Caucasian family with insulin-dependent diabetes mellitus. Tissue Antigens. 1996;47:159–162. [PubMed: 8851734]
- 43.
- Pugliese A, Zeller M, Fernandez A Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. [PubMed: 9054945]
- 44.
- Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. [PubMed: 9054944]
- 45.
- Nerup J, Platz P, Andersen OO, Christy M, Lyngsoe J, Poulsen JE, Ryder LP, Nielsen LS, Thomsen M, Svejgaard A. HL-A antigens and diabetes mellitus. Lancet. 1974;2:864–866. [PubMed: 4137711]
- 46.
- Pociot F. Insulin-dependent diabetes mellitus--a polygenic disorder? Dan Med Bull. 1996;43:216–248. [PubMed: 8813453]
- 47.
- Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–183. [PubMed: 6363172]
- 48.
- Julier C, Hyer RN, Davies J, Merlin F, Soularue P, Briant L, Cathelineau G, Deschamps I, Rotter JI, Froguel P, et al. Insulin-IGF2 region on chromosome 11p encodes a gene implicated in HLA-DR4-dependent diabetes susceptibility. Nature. 1991;354:155–159. [PubMed: 1944595]
- 49.
- Field LL, Tobias R, Magnus T. A locus on chromosome 15q26 (IDDM3) produces susceptibility to insulin-dependent diabetes mellitus. Nat Genet. 1994;8:189–194. [PubMed: 7842018]
- 50.
- Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, Reed PW, Gough SC, Jenkins SC, Palmer SM, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371:130–136. [PubMed: 8072542]
- 51.
- Hashimoto L, Habita C, Beressi JP, Delepine M, Besse C, Cambon-Thomsen A, Deschamps I, Rotter JI, Djoulah S, James MR, et al. Genetic mapping of a susceptibility locus for insulin-dependent diabetes mellitus on chromosome 11q. Nature. 1994;371:161–164. [PubMed: 8072544]
- 52.
- Delepine M, Pociot F, Habita C, Hashimoto L, Froguel P, Rotter J, Cambon-Thomsen A, Deschamps I, Djoulah S, Weissenbach J, Nerup J, Lathrop M, Julier C. Evidence of a non-MHC susceptibility locus in type I diabetes linked to HLA on chromosome 6. Am J Hum Genet. 1997;60:174–187. [PMC free article: PMC1712534] [PubMed: 8981961]
- 53.
- Merriman T, Twells R, Merriman M, Eaves I, Cox R, Cucca F, McKinney P, Shield J, Baum D, Bosi E, Pozzilli P, Nistico L, Buzzetti R, Joner G, Ronningen KS, Thorsby E, Undlien D, Pociot F, Nerup J, Bain S, Barnett A, Todd J. Evidence by allelic association-dependent methods for a type 1 diabetes polygene (IDDM6) on chromosome 18q21. Hum Mol Genet. 1997;6:1003–1010. [PubMed: 9215667]
- 54.
- Owerbach D, Gabbay KH. The HOXD8 locus (2q31) is linked to type I diabetes. Interaction with chromosome 6 and 11 disease susceptibility genes. Diabetes. 1995;44:132–136. [PubMed: 7813807]
- 55.
- Mein CA, Esposito L, Dunn MG, Johnson GC, Timms AE, Goy JV, Smith AN, Sebag-Montefiore L, Merriman ME, Wilson AJ, Pritchard LE, Cucca F, Barnett AH, Bain SC, Todd JA. A search for type 1 diabetes susceptibility genes in families from the United Kingdom. Nat Genet. 1998;19:297–300. [PubMed: 9662409]
- 56.
- Reed P, Cucca F, Jenkins S, Merriman M, Wilson A, McKinney P, Bosi E, Joner G, Ronningen KS, Thorsby E, Undlien D, Merriman T, Barnett A, Bain S, Todd J. Evidence for a type 1 diabetes susceptibility locus (IDDM10) on human chromosome 10p11-q11. Hum Mol Genet. 1997;6:1011–1016. [PubMed: 9215668]
- 57.
- Field LL, Tobias R, Thomson G, Plon S. Susceptibility to insulin-dependent diabetes mellitus maps to a locus (IDDM11) on human chromosome 14q24.3-q31. Genomics. 1996;33:1–8. [PubMed: 8617492]
- 58.
- Copeman JB, Cucca F, Hearne CM, Cornall RJ, Reed PW, Ronningen KS, Undlien DE, Nistico L, Buzzetti R, Tosi R, et al. Linkage disequilibrium mapping of a type 1 diabetes susceptibility gene (IDDM7) to chromosome 2q31-q33. Nat Genet. 1995;9:80–85. [PubMed: 7704030]
- 59.
- Morahan G, Huang D, Tait BD, Colman PG, Harrison LC. Markers on distal chromosome 2q linked to insulin-dependent diabetes mellitus. Science. 1996;272:1811–1813. [PubMed: 8650584]
- 60.
- Verge CF, Vardi P, Babu S, Bao F, Erlich HA, Bugawan T, Tiosano D, Yu L, Eisenbarth GS, Fain PR. Evidence for oligogenic inheritance of type 1 diabetes in a large Bedouin Arab family. J Clin Invest. 1998;102:1569–1575. [PMC free article: PMC509007] [PubMed: 9788970]
- 61.
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. [PubMed: 10932183]
- 62.
- Nerup J, Pociot F. European Consortium for IS. A genomewide scan for type 1-diabetes susceptibility in Scandinavian families: identification of new loci with evidence of interactions. Am J Hum Genet. 2001;69:1301–1313. [PMC free article: PMC1235542] [PubMed: 11598829]
- 63.
- Concannon P, Gogolin-Ewens KJ, Hinds DA, Wapelhorst B, Morrison VA, Stirling B, Mitra M, Farmer J, Williams SR, Cox NJ, Bell GI, Risch N, Spielman RS. A second-generation screen of the human genome for susceptibility to insulin-dependent diabetes mellitus. Nat Genet. 1998;19:292–296. [PubMed: 9662408]
- 64.
- Cucca F, Goy JV, Kawaguchi Y, Esposito L, Merriman ME, Wilson AJ, Cordell HJ, Bain SC, Todd JA. A male-female bias in type 1 diabetes and linkage to chromosome Xp in MHC HLA-DR3-positive patients. Nat Genet. 1998;19:301–302. [PubMed: 9662410]
- 65.
- Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, Farber E, Bonnie JK, Szpak M, Schofield E, Achuthan P, Guo H, Fortune MD, Stevens H, Walker NM, Ward LD, Kundaje A, Kellis M, Daly MJ, Barrett JC, Cooper JD, Deloukas P. Type 1 Diabetes Genetics C, Todd JA, Wallace C, Concannon P, Rich SS. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–386. [PMC free article: PMC4380767] [PubMed: 25751624]
- 66.
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Type 1 Diabetes Genetics C. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. [PMC free article: PMC2889014] [PubMed: 19430480]
- 67.
- Eisenbarth GS. Banting Lecture 2009: An unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes. 2010;59:759–774. [PMC free article: PMC2844823] [PubMed: 20350969]
- 68.
- Krogvold L, Edwin B, Buanes T, Frisk G, Skog O, Anagandula M, Korsgren O, Undlien D, Eike MC, Richardson SJ, Leete P, Morgan NG, Oikarinen S, Oikarinen M, Laiho JE, Hyoty H, Ludvigsson J, Hanssen KF, Dahl-Jorgensen K. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64:1682–1687. [PubMed: 25422108]
- 69.
- Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jorgensen K, von Herrath M, Pugliese A, Atkinson MA, Morgan NG. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59:2448–2458. [PMC free article: PMC5042874] [PubMed: 27506584]
- 70.
- Lundberg M, Krogvold L, Kuric E, Dahl-Jorgensen K, Skog O. Expression of Interferon-Stimulated Genes in Insulitic Pancreatic Islets of Patients Recently Diagnosed With Type 1 Diabetes. Diabetes. 2016;65:3104–3110. [PubMed: 27422384]
- 71.
- Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1283. [PubMed: 4139522]
- 72.
- Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9:154–162. [PubMed: 6990358]
- 73.
- Huang W, Connor E, Rosa TD, Muir A, Schatz D, Silverstein J, Crockett S, She JX, Maclaren NK. Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in beta-cell autoimmunity. J Clin Endocrinol Metab. 1996;81:2559–2563. [PubMed: 8675578]
- 74.
- Maclaren NK, Huang SW, Fogh J. Antibody to cultured human insulinoma cells in insulin-dependent diabetes. Lancet. 1975;1:997–1000. [PubMed: 48725]
- 75.
- Neufeld M, Maclaren NK, Riley WJ, Lezotte D, McLaughlin JV, Silverstein J, Rosenbloom AL. Islet cell and other organ-specific antibodies in U.S. Caucasians and Blacks with insulin-dependent diabetes mellitus. Diabetes. 1980;29:589–592. [PubMed: 7002675]
- 76.
- Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. [PubMed: 6362005]
- 77.
- Atkinson MA, Maclaren NK, Riley WJ, Winter WE, Fisk DD, Spillar RP. Are insulin autoantibodies markers for insulin-dependent diabetes mellitus? Diabetes. 1986;35:894–898. [PubMed: 3525287]
- 78.
- Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156. [PubMed: 1697648]
- 79.
- Atkinson MA, Kaufman DL, Newman D, Tobin AJ, Maclaren NK. Islet cell cytoplasmic autoantibody reactivity to glutamate decarboxylase in insulin-dependent diabetes. J Clin Invest. 1993;91:350–356. [PMC free article: PMC330033] [PubMed: 8423231]
- 80.
- Christie MR, Genovese S, Cassidy D, Bosi E, Brown TJ, Lai M, Bonifacio E, Bottazzo GF. Antibodies to islet 37k antigen, but not to glutamate decarboxylase, discriminate rapid progression to IDDM in endocrine autoimmunity. Diabetes. 1994;43:1254–1259. [PubMed: 7926297]
- 81.
- Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, Notkins AL, Lan MS. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. 1996;93:2307–2311. [PMC free article: PMC39791] [PubMed: 8637868]
- 82.
- Solimena M, Dirkx R Jr, Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, Rabin DU. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996;15:2102–2114. [PMC free article: PMC450132] [PubMed: 8641276]
- 83.
- Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1996;93:6367–6370. [PMC free article: PMC39028] [PubMed: 8692821]
- 84.
- Notkins AL, Lu J, Li Q, VanderVegt FP, Wasserfall C, Maclaren NK, Lan MS. IA-2 and IA-2 beta are major autoantigens in IDDM and the precursors of the 40 kDa and 37 kDa tryptic fragments. J Autoimmun. 1996;9:677–682. [PubMed: 8933284]
- 85.
- Lan MS, Maclaren NK. Cryptic epitope and autoimmunity. Diabetes Metab Rev. 1998;14:333–334. [PubMed: 10096004]
- 86.
- Aanstoot HJ, Kang SM, Kim J, Lindsay LA, Roll U, Knip M, Atkinson M, Mose-Larsen P, Fey S, Ludvigsson J, Landin L, Bruining J, Maclaren N, Akerblom HK, Baekkeskov S. Identification and characterization of glima 38, a glycosylated islet cell membrane antigen, which together with GAD65 and IA2 marks the early phases of autoimmune response in type 1 diabetes. J Clin Invest. 1996;97:2772–2783. [PMC free article: PMC507370] [PubMed: 8675688]
- 87.
- Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EA, Bottazzo GF. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335:147–149. [PubMed: 1967440]
- 88.
- Riley WJ, Atkinson MA, Schatz DA, Maclaren NK. Comparison of islet autoantibodies in 'pre-diabetes' and recommendations for screening. J Autoimmun. 1990;3 Suppl 1:47–51. [PubMed: 2187460]
- 89.
- Krischer JP, Schatz D, Riley WJ, Spillar RP, Silverstein JH, Schwartz S, Malone J, Shah S, Vadheim CM, Rotter JI, et al. Insulin and islet cell autoantibodies as time-dependent covariates in the development of insulin-dependent diabetes: a prospective study in relatives. J Clin Endocrinol Metab. 1993;77:743–749. [PubMed: 8370696]
- 90.
- Irvine WJ, McCallum CJ, Gray RS, Campbell CJ, Duncan LJ, Farquhar JW, Vaughan H, Morris PJ. Pancreatic islet-cell antibodies in diabetes mellitus correlated with the duration and type of diabetes, coexistent autoimmune disease, and HLA type. Diabetes. 1977;26:138–147. [PubMed: 320073]
- 91.
- Riley WJ, Maclaren NK, Krischer J, Spillar RP, Silverstein JH, Schatz DA, Schwartz S, Malone J, Shah S, Vadheim C, et al. A prospective study of the development of diabetes in relatives of patients with insulin-dependent diabetes. N Engl J Med. 1990;323:1167–1172. [PubMed: 2215594]
- 92.
- Roll U, Christie MR, Fuchtenbusch M, Payton MA, Hawkes CJ, Ziegler AG. Perinatal autoimmunity in offspring of diabetic parents. The German Multicenter BABY-DIAB study: detection of humoral immune responses to islet antigens in early childhood. Diabetes. 1996;45:967–973. [PubMed: 8666150]
- 93.
- Hamalainen AM, Ronkainen MS, Akerblom HK, Knip M. Postnatal elimination of transplacentally acquired disease-associated antibodies in infants born to families with type 1 diabetes. The Finnish TRIGR Study Group. Trial to Reduce IDDM in the Genetically at Risk. J Clin Endocrinol Metab. 2000;85:4249–4253. [PubMed: 11095462]
- 94.
- Koczwara K, Bonifacio E, Ziegler AG. Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes. 2004;53:1–4. [PubMed: 14693690]
- 95.
- Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. [PubMed: 10078544]
- 96.
- Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–1403. [PubMed: 10651256]
- 97.
- Kumar V, Sercarz E. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J Immunol. 1998;161:6585–6591. [PubMed: 9862685]
- 98.
- Irvine WJ, Clarke BF, Scarth L, Cullen DR, Duncan LJ. Thyroid and gastric autoimmunity in patients with diabetes mellitus. Lancet. 1970;2:163–168. [PubMed: 4193564]
- 99.
- Riley WJ, Maclaren NK, Neufeld M. Adrenal autoantibodies and Addison disease in insulin-dependent diabetes mellitus. J Pediatr. 1980;97:191–195. [PubMed: 7400884]
- 100.
- Riley WJ, Toskes PP, Maclaren NK, Silverstein JH. Predictive value of gastric parietal cell autoantibodies as a marker for gastric and hematologic abnormalities associated with insulin-dependent diabetes. Diabetes. 1982;31:1051–1055. [PubMed: 7173496]
- 101.
- Rapoport MJ, Bistritzer T, Vardi O, Broide E, Azizi A, Vardi P. Increased prevalence of diabetes-related autoantibodies in celiac disease. J Pediatr Gastroenterol Nutr. 1996;23:524–527. [PubMed: 8985839]
- 102.
- Song YH, Connor E, Li Y, Zorovich B, Balducci P, Maclaren N. The role of tyrosinase in autoimmune vitiligo. Lancet. 1994;344:1049–1052. [PubMed: 7934446]
- 103.
- Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–2129. [PMC free article: PMC294659] [PubMed: 7962558]
- 104.
- Ellis TM, Atkinson MA. The clinical significance of an autoimmune response against glutamic acid decarboxylase. Nat Med. 1996;2:148–153. [PubMed: 8574952]
- 105.
- Ellis TM, Schatz DA, Ottendorfer EW, Lan MS, Wasserfall C, Salisbury PJ, She JX, Notkins AL, Maclaren NK, Atkinson MA. The relationship between humoral and cellular immunity to IA-2 in IDDM. Diabetes. 1998;47:566–569. [PubMed: 9568688]
- 106.
- Bach JF, Chatenoud L. Immunosuppression in insulin-dependent diabetes mellitus: from cellular selectivity towards autoantigen specificity. Chem Immunol. 1995;60:32–47. [PubMed: 7531979]
- 107.
- Atkinson MA, Kaufman DL, Campbell L, Gibbs KA, Shah SC, Bu DF, Erlander MG, Tobin AJ, Maclaren NK. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992;339:458–459. [PubMed: 1346821]
- 108.
- Harrison LC, Honeyman MC, DeAizpurua HJ, Schmidli RS, Colman PG, Tait BD, Cram DS. Inverse relation between humoral and cellular immunity to glutamic acid decarboxylase in subjects at risk of insulin-dependent diabetes. Lancet. 1993;341:1365–1369. [PubMed: 8098789]
- 109.
- Semana G, Gausling R, Jackson RA, Hafler DA. T cell autoreactivity to proinsulin epitopes in diabetic patients and healthy subjects. J Autoimmun. 1999;12:259–267. [PubMed: 10330297]
- 110.
- Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottlieb PA, Putnam AL, Gaur A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–180. [PMC free article: PMC198872] [PubMed: 11160133]
- 111.
- Roep BO, Atkinson MA, van Endert PM, Gottlieb PA, Wilson SB, Sachs JA. Autoreactive T cell responses in insulin-dependent (Type 1) diabetes mellitus. Report of the first international workshop for standardization of T cell assays. J Autoimmun. 1999;13:267–282. [PubMed: 10479395]
- 112.
- Endl J, Otto H, Jung G, Dreisbusch B, Donie F, Stahl P, Elbracht R, Schmitz G, Meinl E, Hummel M, Ziegler AG, Wank R, Schendel DJ. Identification of naturally processed T cell epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J Clin Invest. 1997;99:2405–2415. [PMC free article: PMC508080] [PubMed: 9153283]
- 113.
- Honeyman MC, Stone N, de Aizpurua H, Rowley MJ, Harrison LC. High T cell responses to the glutamic acid decarboxylase (GAD) isoform 67 reflect a hyperimmune state that precedes the onset of insulin-dependent diabetes. J Autoimmun. 1997;10:165–173. [PubMed: 9185878]
- 114.
- Lohmann T, Leslie RD, Hawa M, Geysen M, Rodda S, Londei M. Immunodominant epitopes of glutamic acid decarboxylase 65 and 67 in insulin-dependent diabetes mellitus. Lancet. 1994;343:1607–1608. [PubMed: 7911924]
- 115.
- Lohmann T, Leslie RD, Londei M. T cell clones to epitopes of glutamic acid decarboxylase 65 raised from normal subjects and patients with insulin-dependent diabetes. J Autoimmun. 1996;9:385–389. [PubMed: 8816975]
- 116.
- Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, Spinas GA, Sinigaglia F. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995;181:1923–1927. [PMC free article: PMC2191981] [PubMed: 7722468]
- 117.
- Weiss U, Manfras BJ, Terjung D, Eiermann T, Wolpl A, Loliger C, Kuhnl P, Boehm BO. In vitro stimulation with glutamic acid decarboxylase (GAD65) leads to an oligoclonal response of peripheral T-cells in an IDDM patient. Scand J Immunol. 1995;42:673–678. [PubMed: 8552991]
- 118.
- Pugliese A, Yang M, Kusmarteva I, Heiple T, Vendrame F, Wasserfall C, Rowe P, Moraski JM, Ball S, Jebson L, Schatz DA, Gianani R, Burke GW, Nierras C, Staeva T, Kaddis JS, Campbell-Thompson M, Atkinson MA. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15:1–9. [PMC free article: PMC4282794] [PubMed: 24325575]
- 119.
- Krogvold L, Skog O, Sundstrom G, Edwin B, Buanes T, Hanssen KF, Ludvigsson J, Grabherr M, Korsgren O, Dahl-Jorgensen K. Function of Isolated Pancreatic Islets From Patients at Onset of Type 1 Diabetes: Insulin Secretion Can Be Restored After Some Days in a Nondiabetogenic Environment In Vitro: Results From the DiViD Study. Diabetes. 2015;64:2506–2512. [PubMed: 25677915]
- 120.
- Campbell-Thompson M. Organ donor specimens: What can they tell us about type 1 diabetes? Pediatr Diabetes. 2015;16:320–330. [PMC free article: PMC4718555] [PubMed: 25998576]
- 121.
- Michels A, Zhang L, Khadra A, Kushner JA, Redondo MJ, Pietropaolo M. Prediction and prevention of type 1 diabetes: update on success of prediction and struggles at prevention. Pediatr Diabetes. 2015;16:465–484. [PMC free article: PMC4592445] [PubMed: 26202050]
- 122.
- Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993;36:883–892. [PubMed: 8243865]
- 123.
- Marron MP, Raffel LJ, Garchon HJ, Jacob CO, Serrano-Rios M, Martinez Larrad MT, Teng WP, Park Y, Zhang ZX, Goldstein DR, Tao YW, Beaurain G, Bach JF, Huang HS, Luo DF, Zeidler A, Rotter JI, Yang MC, Modilevsky T, Maclaren NK, She JX. Insulin-dependent diabetes mellitus (IDDM) is associated with CTLA4 polymorphisms in multiple ethnic groups. Hum Mol Genet. 1997;6:1275–1282. [PubMed: 9259273]
- 124.
- See DM, Tilles JG. The pathogenesis of viral-induced diabetes. Clin Diagn Virol. 1998;9:85–88. [PubMed: 9645989]
- 125.
- Vreugdenhil GR, Geluk A, Ottenhoff TH, Melchers WJ, Roep BO, Galama JM. Molecular mimicry in diabetes mellitus: the homologous domain in coxsackie B virus protein 2C and islet autoantigen GAD65 is highly conserved in the coxsackie B-like enteroviruses and binds to the diabetes associated HLA-DR3 molecule. Diabetologia. 1998;41:40–46. [PubMed: 9498628]
- 126.
- Srinivasappa J, Saegusa J, Prabhakar BS, Gentry MK, Buchmeier MJ, Wiktor TJ, Koprowski H, Oldstone MB, Notkins AL. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986;57:397–401. [PMC free article: PMC252745] [PubMed: 3753614]
- 127.
- Jenson AB, Rosenberg HS, Notkins AL. Pancreatic islet-cell damage in children with fatal viral infections. Lancet. 1980;2:354–358. [PubMed: 6105486]
- 128.
- Yoon JW, Onodera T, Notkins AL. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with coxsackie virus B4. J Exp Med. 1978;148:1068–1080. [PMC free article: PMC2185018] [PubMed: 212506]
- 129.
- Yoon JW, Selvaggio S, Onodera T, Wheeler J, Jenson AB. Infection of cultured human pancreatic B cells with reovirus type 3. Diabetologia. 1981;20:462–467. [PubMed: 7016640]
- 130.
- Prince GA, Jenson AB, Billups LC, Notkins AL. Infection of human pancreatic beta cell cultures with mumps virus. Nature. 1978;271:158–161. [PubMed: 340957]
- 131.
- Parkkonen P, Hyoty H, Koskinen L, Leinikki P. Mumps virus infects beta cells in human fetal islet cell cultures upregulating the expression of HLA class I molecules. Diabetologia. 1992;35:63–69. [PubMed: 1311693]
- 132.
- Yoon JW, Onodera T, Jenson AB, Notkins AL. Virus-induced diabetes mellitus. XI. Replication of coxsackie B3 virus in human pancreatic beta cell cultures. Diabetes. 1978;27:778–781. [PubMed: 350678]
- 133.
- Campbell IL, Harrison LC, Ashcroft RG, Jack I. Reovirus infection enhances expression of class I MHC proteins on human beta-cell and rat RINm5F cell. Diabetes. 1988;37:362–365. [PubMed: 2836251]
- 134.
- Pak CY, Eun HM, McArthur RG, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2:1–4. [PubMed: 2898620]
- 135.
- Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:283–292. [PMC free article: PMC442846] [PubMed: 1370298]
- 136.
- Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. [PMC free article: PMC8216222] [PubMed: 7694152]
- 137.
- Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. [PubMed: 8232539]
- 138.
- Tian J, Lehmann PV, Kaufman DL. T cell cross-reactivity between coxsackievirus and glutamate decarboxylase is associated with a murine diabetes susceptibility allele. J Exp Med. 1994;180:1979–1984. [PMC free article: PMC2191714] [PubMed: 7964474]
- 139.
- Szopa TM, Titchener PA, Portwood ND, Taylor KW. Diabetes mellitus due to viruses--some recent developments. Diabetologia. 1993;36:687–695. [PubMed: 8405735]
- 140.
- Schloot NC, Roep BO, Wegmann DR, Yu L, Wang TB, Eisenbarth GS. T-cell reactivity to GAD65 peptide sequences shared with coxsackie virus protein in recent-onset IDDM, post-onset IDDM patients and control subjects. Diabetologia. 1997;40:332–338. [PubMed: 9084973]
- 141.
- Honeyman MC, Stone NL, Harrison LC. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol Med. 1998;4:231–239. [PMC free article: PMC2230363] [PubMed: 9606176]
- 142.
- Sutkowski N, Palkama T, Ciurli C, Sekaly RP, Thorley-Lawson DA, Huber BT. An Epstein-Barr virus-associated superantigen. J Exp Med. 1996;184:971–980. [PMC free article: PMC2192769] [PubMed: 9064357]
- 143.
- White J, Herman A, Pullen AM, Kubo R, Kappler JW, Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. [PubMed: 2521300]
- 144.
- Hao W, Serreze DV, McCulloch DK, Neifing JL, Palmer JP. Insulin (auto)antibodies from human IDDM cross-react with retroviral antigen p73. J Autoimmun. 1993;6:787–798. [PubMed: 8155257]
- 145.
- Conrad B, Weissmahr RN, Boni J, Arcari R, Schupbach J, Mach B. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90:303–313. [PubMed: 9244304]
- 146.
- Conrad B, Weidmann E, Trucco G, Rudert WA, Behboo R, Ricordi C, Rodriquez-Rilo H, Finegold D, Trucco M. Evidence for superantigen involvement in insulin-dependent diabetes mellitus aetiology. Nature. 1994;371:351–355. [PubMed: 8090207]
- 147.
- Lan MS, Mason A, Coutant R, Chen QY, Vargas A, Rao J, Gomez R, Chalew S, Garry R, Maclaren NK. HERV-K10s and immune-mediated (type 1) diabetes. Cell. 1998;95:14–16. [PubMed: 9778243]
- 148.
- Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–208. [PubMed: 7686009]
- 149.
- Serreze DV, Leiter EH, Kuff EL, Jardieu P, Ishizaka K. Molecular mimicry between insulin and retroviral antigen p73. Development of cross-reactive autoantibodies in sera of NOD and C57BL/KsJ db/db mice. Diabetes. 1988;37:351–358. [PubMed: 3286334]
- 150.
- Naserke HE, Ziegler AG, Lampasona V, Bonifacio E. Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J Immunol. 1998;161:6963–6969. [PubMed: 9862731]
- 151.
- Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, Strominger JL, Hafler DA. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391:177–181. [PubMed: 9428763]
- 152.
- Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. [PMC free article: PMC150819] [PubMed: 11781358]
- 153.
- Baxter AG, Kinder SJ, Hammond KJ, Scollay R, Godfrey DI. Association between alphabetaTCR+CD4-CD8- T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–582. [PubMed: 9075796]
- 154.
- Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–1056. [PMC free article: PMC2212199] [PubMed: 9529321]
- 155.
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. [PubMed: 10795741]
- 156.
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. [PMC free article: PMC4729454] [PubMed: 26606968]
- 157.
- Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, Lee J, Bluestone JA, Anderson M, Herold KC. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes. 2018;67:1471–1480. [PMC free article: PMC6054443] [PubMed: 29937434]
- 158.
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–168. [PubMed: 29320654]
- 159.
- June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med. 2017;23:540–547. [PubMed: 28475571]
- 160.
- Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304:1020–1022. [PMC free article: PMC1881717] [PubMed: 1586783]
- 161.
- Wilberz S, Partke HJ, Dagnaes-Hansen F, Herberg L. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia. 1991;34:2–5. [PubMed: 1647335]
- 162.
- Takei I, Asaba Y, Kasatani T, Maruyama T, Watanabe K, Yanagawa T, Saruta T, Ishii T. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun. 1992;5:665–673. [PubMed: 1489482]
- 163.
- Martins TC, Aguas AP. Mechanisms of Mycobacterium avium-induced resistance against insulin-dependent diabetes mellitus (IDDM) in non-obese diabetic (NOD) mice: role of Fas and Th1 cells. Clin Exp Immunol. 1999;115:248–254. [PMC free article: PMC1905159] [PubMed: 9933449]
- 164.
- Oldstone MB. Viruses as therapeutic agents. I. Treatment of nonobese insulin-dependent diabetes mice with virus prevents insulin-dependent diabetes mellitus while maintaining general immune competence. J Exp Med. 1990;171:2077–2089. [PMC free article: PMC2187941] [PubMed: 1972180]
- 165.
- Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. [PubMed: 10320614]
- 166.
- Akerblom HK, Knip M. Putative environmental factors in Type 1 diabetes. Diabetes Metab Rev. 1998;14:31–67. [PubMed: 9605629]
- 167.
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. [PubMed: 11476858]
- 168.
- Dahl-Jorgensen K, Joner G, Hanssen KF. Relationship between cows' milk consumption and incidence of IDDM in childhood. Diabetes Care. 1991;14:1081–1083. [PubMed: 1797491]
- 169.
- Scott FW. Cow milk and insulin-dependent diabetes mellitus: is there a relationship? Am J Clin Nutr. 1990;51:489–491. [PubMed: 2309656]
- 170.
- Patterson CC, Dahlquist G, Soltesz G, Green A, Europe EASG. Diabetes. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44 Suppl 3:B9–16. [PubMed: 11724424]
- 171.
- Fava D, Leslie RD, Pozzilli P. Relationship between dairy product consumption and incidence of IDDM in childhood in Italy. Diabetes Care. 1994;17:1488–1490. [PubMed: 7882824]
- 172.
- Norris JM, Beaty B, Klingensmith G, Yu L, Hoffman M, Chase HP, Erlich HA, Hamman RF, Eisenbarth GS, Rewers M. Lack of association between early exposure to cow's milk protein and beta-cell autoimmunity. Diabetes Autoimmunity Study in the Young (DAISY). JAMA. 1996;276:609–614. [PubMed: 8773632]
- 173.
- Kostraba JN, Gay EC, Rewers M, Hamman RF. Nitrate levels in community drinking waters and risk of IDDM. An ecological analysis. Diabetes Care. 1992;15:1505–1508. [PubMed: 1468277]
- 174.
- Virtanen SM, Jaakkola L, Rasanen L, Ylonen K, Aro A, Lounamaa R, Akerblom HK, Tuomilehto J. Nitrate and nitrite intake and the risk for type 1 diabetes in Finnish children. Childhood Diabetes in Finland Study Group. Diabet Med. 1994;11:656–662. [PubMed: 7955990]
- 175.
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. [PubMed: 180605]
- 176.
- Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12:154–167. [PubMed: 26729037]
- 177.
- Neu A, Willasch A, Ehehalt S, Kehrer M, Hub R, Ranke MB. Diabetes incidence in children of different nationalities: an epidemiological approach to the pathogenesis of diabetes. Diabetologia. 2001;44 Suppl 3:B21–26. [PubMed: 11724411]
- 178.
- Maclaren NK, Lan MS, Schatz D, Malone J, Notkins AL, Krischer J. Multiple autoantibodies as predictors of Type 1 diabetes in a general population. Diabetologia. 2003;46:873–874. [PubMed: 12802500]
- 179.
- Samuelsson U, Sundkvist G, Borg H, Fernlund P, Ludvigsson J. Islet autoantibodies in the prediction of diabetes in school children. Diabetes Res Clin Pract. 2001;51:51–57. [PubMed: 11137182]
- 180.
- LaGasse JM, Brantley MS, Leech NJ, Rowe RE, Monks S, Palmer JP, Nepom GT, McCulloch DK, Hagopian WA, Washingtno State Diabetes Prediction S. Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care. 2002;25:505–511. [PubMed: 11874938]
- 181.
- Kimpimaki T, Kulmala P, Savola K, Kupila A, Korhonen S, Simell T, Ilonen J, Simell O, Knip M. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab. 2002;87:4572–4579. [PubMed: 12364437]
- 182.
- Roden M. Wien Klin Wochenschr. 2016;128 Suppl 2:S37–40. [Diabetes mellitus: definition, classification and diagnosis] [PubMed: 27052219]
- 183.
- Beauchamp G, Haller MJ. Can we prevent type 1 diabetes? Curr Diab Rep. 2015;15:86. [PubMed: 26370697]
- 184.
- De Filippo G, Carel JC, Boitard C, Bougneres PF. Long-term results of early cyclosporin therapy in juvenile IDDM. Diabetes. 1996;45:101–104. [PubMed: 8522052]
- 185.
- Carel JC, Boitard C, Eisenbarth G, Bach JF, Bougneres PF. Cyclosporine delays but does not prevent clinical onset in glucose intolerant pre-type 1 diabetic children. J Autoimmun. 1996;9:739–745. [PubMed: 9115576]
- 186.
- European Nicotinamide Diabetes Intervention Trial G. Intervening before the onset of Type 1 diabetes: baseline data from the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetologia. 2003;46:339–346. [PubMed: 12687331]
- 187.
- Lampeter EF, Klinghammer A, Scherbaum WA, Heinze E, Haastert B, Giani G, Kolb H. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes. 1998;47:980–984. [PubMed: 9604880]
- 188.
- Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol. 2013;9:92–103. [PubMed: 23296174]
- 189.
- Muir A, Peck A, Clare-Salzler M, Song YH, Cornelius J, Luchetta R, Krischer J, Maclaren N. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-gamma transcription. J Clin Invest. 1995;95:628–634. [PMC free article: PMC295528] [PubMed: 7860747]
- 190.
- Ramiya VK, Shang XZ, Pharis PG, Wasserfall CH, Stabler TV, Muir AB, Schatz DA, Maclaren NK. Antigen based therapies to prevent diabetes in NOD mice. J Autoimmun. 1996;9:349–356. [PubMed: 8816970]
- 191.
- Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23). Proc Natl Acad Sci U S A. 1996;93:956–960. [PMC free article: PMC40166] [PubMed: 8570667]
- 192.
- Group TS, Akerblom HK, Krischer J, Virtanen SM, Berseth C, Becker D, Dupre J, Ilonen J, Trucco M, Savilahti E, Koski K, Pajakkala E, Fransiscus M, Lough G, Bradley B, Koski M, Knip M. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study: recruitment, intervention and follow-up. Diabetologia. 2011;54:627–633. [PMC free article: PMC3034039] [PubMed: 21153533]
- 193.
- Schmid S, Buuck D, Knopff A, Bonifacio E, Ziegler AG. BABYDIET, a feasibility study to prevent the appearance of islet autoantibodies in relatives of patients with Type 1 diabetes by delaying exposure to gluten. Diabetologia. 2004;47:1130–1131. [PubMed: 15168019]
- 194.
- Chase HP BD, Rodriguez H, Donaldson D, Chritton S, Rafkin-Mervis L, Krischer J, Skyler JS, Clare-Salzler M. Type 1 Diabetes TrialNet Nutritional Intervention to Prevent (NIP) Type 1 Diabetes Study Group. Pediatr Diabetes. 2015;16:271–279. [PMC free article: PMC4291300] [PubMed: 25039804]
- 195.
- Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes--considerations for attempts to prevent and reverse the disease. Diabetes Care. 2015;38:979–988. [PMC free article: PMC4439528] [PubMed: 25998290]
- 196.
- Wicklow BA, Taback SP. Feasibility of a type 1 diabetes primary prevention trial using 2000 IU vitamin D3 in infants from the general population with increased HLA-associated risk. Ann N Y Acad Sci. 2006;1079:310–312. [PubMed: 17130571]
- 197.
- Andersson C, Carlsson A, Cilio C, Cedervall E, Ivarsson SA, Jonsdottir B, Jonsson B, Larsson K, Neiderud J, Lernmark A, Elding Larsson H, Di A-ITSG. Glucose tolerance and beta-cell function in islet autoantibody-positive children recruited to a secondary prevention study. Pediatr Diabetes. 2013;14:341–349. [PubMed: 23469940]
- 198.
- Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, Lecomte P, Marechaud R, Bougneres P, Charbonnel B, Sai P. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–549. [PubMed: 10950231]
- 199.
- Castellana M, Cignarelli A, Brescia F, Perrini S, Natalicchio A, Laviola L, Giorgino F. Efficacy and safety of GLP-1 receptor agonists as add-on to SGLT2 inhibitors in type 2 diabetes mellitus: A meta-analysis. Sci Rep. 2019;9:19351. [PMC free article: PMC6920368] [PubMed: 31852920]
- 200.
- Bonifacio E, Ziegler AG, Klingensmith G, Schober E, Bingley PJ, Rottenkolber M, Theil A, Eugster A, Puff R, Peplow C, Buettner F, Lange K, Hasford J, Achenbach P, Pre PSG. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA. 2015;313:1541–1549. [PubMed: 25898052]
- 201.
- Vaarala O, Ilonen J, Ruohtula T, Pesola J, Virtanen SM, Harkonen T, Koski M, Kallioinen H, Tossavainen O, Poussa T, Jarvenpaa AL, Komulainen J, Lounamaa R, Akerblom HK, Knip M. Removal of Bovine Insulin From Cow's Milk Formula and Early Initiation of Beta-Cell Autoimmunity in the FINDIA Pilot Study. Arch Pediatr Adolesc Med. 2012;166:608–614. [PubMed: 22393174]
- 202.
- Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ. Type 1 Diabetes TrialNet Study G. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019;381:603–613. [PMC free article: PMC6776880] [PubMed: 31180194]
- 203.
- Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, Vercruysse F, Investigators TGS. Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes. N Engl J Med. 2020;383:2007–2017. [PubMed: 33207093]
- 204.
- Shah AS, D'Alessio D, Ford-Adams ME, Desai AP, Inge TH. Bariatric Surgery: A Potential Treatment for Type 2 Diabetes in Youth. Diabetes Care. 2016;39:934–940. [PMC free article: PMC5317244] [PubMed: 27222551]
- 205.
- Dagogo-Jack S. Predicting diabetes: our relentless quest for genomic nuggets. Diabetes Care. 2012;35:193–195. [PMC free article: PMC3263887] [PubMed: 22275439]
- 206.
- Writing Group for the SfDiYSG. Dabelea D, Bell RA, D'Agostino RB, Jr., Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. [PubMed: 17595272]
- 207.
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. [PubMed: 2044434]
- 208.
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. [PubMed: 11809615]
- 209.
- Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37:622–628. [PubMed: 3360218]
- 210.
- Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23:381–389. [PubMed: 10868870]
- 211.
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. [PMC free article: PMC3214617] [PubMed: 17463248]
- 212.
- Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. [PubMed: 8476236]
- 213.
- Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976;294:739–745. [PubMed: 176581]
- 214.
- Ten S, Bhangoo A, Ramchandani N, Mueller C, Vogiatzi M, New M, Lesser M, Maclaren N. Characterization of insulin resistance syndrome in children and young adults. When to screen for prediabetes? J Pediatr Endocrinol Metab. 2007;20:989–999. [PubMed: 18038708]
- 215.
- Chadt A, Scherneck S, Joost HG, Al-Hasani H. Molecular links between Obesity and Diabetes: "Diabesity". In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth (MA)2000.
- 216.
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. [PubMed: 11856796]
- 217.
- Sperling MA, Garg A. Monogenic Forms of Diabetes. In: rd, Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, eds. Diabetes in America. Bethesda (MD)2018. [PubMed: 33651552]
- 218.
- Laffel L, Chang N, Grey M, Hale D, Higgins L, Hirst K, Izquierdo R, Larkin M, Macha C, Pham T, Wauters A, Weinstock RS, Group TS. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes. 2012;13:369–375. [PMC free article: PMC3371323] [PubMed: 22369102]
- 219.
- Allen DB. TODAY--a stark glimpse of tomorrow. N Engl J Med. 2012;366:2315–2316. [PubMed: 22540913]
- 220.
- Galloway PJ, Donaldson MD, Wallace AM. Sex hormone binding globulin concentration as a prepubertal marker for hyperinsulinaemia in obesity. Arch Dis Child. 2001;85:489–491. [PMC free article: PMC1719027] [PubMed: 11719335]
- 221.
- Karnieli E, Cohen P, Barzilai N, Ish-Shalom Z, Armoni M, Rafaelov R, Barzilai D. Insulin resistance in Cushing's syndrome. Horm Metab Res. 1985;17:518–521. [PubMed: 3905556]
- 222.
- Low L, Chernausek SD, Sperling MA. Acromegaloid patients with type A insulin resistance: parallel defects in insulin and insulin-like growth factor-I receptors and biological responses in cultured fibroblasts. J Clin Endocrinol Metab. 1989;69:329–337. [PubMed: 2546962]
- 223.
- Rogers DL. Acanthosis nigricans. Semin Dermatol. 1991;10:160–163. [PubMed: 1931564]
- 224.
- Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. [PubMed: 7555456]
- 225.
- Kawasaki T, Hashimoto N, Kikuchi T, Takahashi H, Uchiyama M. The relationship between fatty liver and hyperinsulinemia in obese Japanese children. J Pediatr Gastroenterol Nutr. 1997;24:317–321. [PubMed: 9138179]
- 226.
- Tazawa Y, Noguchi H, Nishinomiya F, Takada G. Serum alanine aminotransferase activity in obese children. Acta Paediatr. 1997;86:238–241. [PubMed: 9099310]
- 227.
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. [PubMed: 11567710]
- 228.
- Fan Y, Fang X, Tajima A, Geng X, Ranganathan S, Dong H, Trucco M, Sperling MA. Evolution of hepatic steatosis to fibrosis and adenoma formation in liver-specific growth hormone receptor knockout mice. Front Endocrinol (Lausanne). 2014;5:218. [PMC free article: PMC4270248] [PubMed: 25566190]
- 229.
- Reinehr T, Isa A, de Sousa G, Dieffenbach R, Andler W. Thyroid hormones and their relation to weight status. Horm Res. 2008;70:51–57. [PubMed: 18493150]
- 230.
- Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. [PubMed: 19540303]
- 231.
- Reinehr T. Thyroid function in the nutritionally obese child and adolescent. Curr Opin Pediatr. 2011;23:415–420. [PubMed: 21430532]
- 232.
- Sperling MA GA. Monogenic Forms of Diabetes. Diabetes in America. 3rd ed2016:7-1-7-27.
- 233.
- Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878–1884. [PMC free article: PMC3142024] [PubMed: 21788644]
- 234.
- Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971–980. [PubMed: 11575290]
- 235.
- Pihoker C, Gilliam LK, Ellard S, Dabelea D, Davis C, Dolan LM, Greenbaum CJ, Imperatore G, Lawrence JM, Marcovina SM, Mayer-Davis E, Rodriguez BL, Steck AK, Williams DE, Hattersley AT. Group SfDiYS. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98:4055–4062. [PMC free article: PMC3790621] [PubMed: 23771925]
- 236.
- Ellard S, Bellanne-Chantelot C, Hattersley AT., European Molecular Genetics Quality Network Mg. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546–553. [PMC free article: PMC2270360] [PubMed: 18297260]
- 237.
- Dussoix P, Vaxillaire M, Iynedjian PB, Tiercy JM, Ruiz J, Spinas GA, Berger W, Zahnd G, Froguel P, Philippe J. Diagnostic heterogeneity of diabetes in lean young adults: classification based on immunological and genetic parameters. Diabetes. 1997;46:622–631. [PubMed: 9075802]
- 238.
- Tuomi T, Miettinen PJ, Hakaste L, Groop L. Atypical Forms of Diabetes. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth (MA)2000.
- 239.
- Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504–2508. [PubMed: 20499044]
- 240.
- Chakera AJ, Steele AM, Gloyn AL, Shepherd MH, Shields B, Ellard S, Hattersley AT. Recognition and Management of Individuals With Hyperglycemia Because of a Heterozygous Glucokinase Mutation. Diabetes Care. 2015;38:1383–1392. [PubMed: 26106223]
- 241.
- Hattersley AT, Greeley SAW, Polak M, Rubio-Cabezas O, Njolstad PR, Mlynarski W, Castano L, Carlsson A, Raile K, Chi DV, Ellard S, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:47–63. [PubMed: 30225972]
- 242.
- Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1). Nature. 1996;384:458–460. [PubMed: 8945471]
- 243.
- Froguel P, Zouali H, Vionnet N, Velho G, Vaxillaire M, Sun F, Lesage S, Stoffel M, Takeda J, Passa P, et al. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med. 1993;328:697–702. [PubMed: 8433729]
- 244.
- Hansen T, Eiberg H, Rouard M, Vaxillaire M, Moller AM, Rasmussen SK, Fridberg M, Urhammer SA, Holst JJ, Almind K, Echwald SM, Hansen L, Bell GI, Pedersen O. Novel MODY3 mutations in the hepatocyte nuclear factor-1alpha gene: evidence for a hyperexcitability of pancreatic beta-cells to intravenous secretagogues in a glucose-tolerant carrier of a P447L mutation. Diabetes. 1997;46:726–730. [PubMed: 9075819]
- 245.
- Peters AL, Davidson MB, Schriger DL, Hasselblad V. A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels. Meta-analysis Research Group on the Diagnosis of Diabetes Using Glycated Hemoglobin Levels. JAMA. 1996;276:1246–1252. [PubMed: 8849753]
- 246.
- Pontoglio M, Prie D, Cheret C, Doyen A, Leroy C, Froguel P, Velho G, Yaniv M, Friedlander G. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000;1:359–365. [PMC free article: PMC1083745] [PubMed: 11269503]
- 247.
- Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. [PubMed: 9326926]
- 248.
- Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. [PubMed: 9398836]
- 249.
- Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. [PMC free article: PMC316513] [PubMed: 9308961]
- 250.
- Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, Vaillant E, Benmezroua Y, Durand E, Bakaher N, Delannoy V, Vaxillaire M, Cook T, Dallinga-Thie GM, Jansen H, Charles MA, Clement K, Galan P, Hercberg S, Helbecque N, Charpentier G, Prentki M, Hansen T, Pedersen O, Urrutia R, Melloul D, Froguel P. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102:4807–4812. [PMC free article: PMC554843] [PubMed: 15774581]
- 251.
- Raeder H, Johansson S, Holm PI, Haldorsen IS, Mas E, Sbarra V, Nermoen I, Eide SA, Grevle L, Bjorkhaug L, Sagen JV, Aksnes L, Sovik O, Lombardo D, Molven A, Njolstad PR. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38:54–62. [PubMed: 16369531]
- 252.
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. [PubMed: 9163426]
- 253.
- Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Stoy J, Steiner DF, Philipson LH, Bell GI. Neonatal Diabetes International Collaborative G, Hattersley AT, Ellard S. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57:1034–1042. [PMC free article: PMC7611804] [PubMed: 18162506]
- 254.
- Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, El Khattabi I, Kim SH, Marselli L, Rich SS, Krolewski AS, Bonner-Weir S, Sharma A, Sale M, Mychaleckyj JC, Kulkarni RN, Doria A. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:14460–14465. [PMC free article: PMC2732833] [PubMed: 19667185]
- 255.
- Bonnefond A, Froguel P. Rare and common genetic events in type 2 diabetes: what should biologists know? Cell Metab. 2015;21:357–368. [PubMed: 25640731]
- 256.
- Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60:769–777. [PMC free article: PMC5907633] [PubMed: 28314945]
- 257.
- Prudente S, Jungtrakoon P, Marucci A, Ludovico O, Buranasupkajorn P, Mazza T, Hastings T, Milano T, Morini E, Mercuri L, Bailetti D, Mendonca C, Alberico F, Basile G, Romani M, Miccinilli E, Pizzuti A, Carella M, Barbetti F, Pascarella S, Marchetti P, Trischitta V, Di Paola R, Doria A. Loss-of-Function Mutations in APPL1 in Familial Diabetes Mellitus. Am J Hum Genet. 2015;97:177–185. [PMC free article: PMC4571002] [PubMed: 26073777]
- 258.
- De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, Ellard S, Hattersley AT. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386:957–963. [PMC free article: PMC4772451] [PubMed: 26231457]
- 259.
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT. Neonatal Diabetes International Collaborative G. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. [PubMed: 16885550]
- 260.
- Johns DR. Seminars in medicine of the Beth Israel Hospital, Boston. Mitochondrial DNA and disease. N Engl J Med. 1995;333:638–644. [PubMed: 7637726]
- 261.
- Robbins DC, Shoelson SE, Rubenstein AH, Tager HS. Familial hyperproinsulinemia. Two cohorts secreting indistinguishable type II intermediates of proinsulin conversion. J Clin Invest. 1984;73:714–719. [PMC free article: PMC425073] [PubMed: 6368587]
- 262.
- Nishi M, Nanjo K. Insulin gene mutations and diabetes. J Diabetes Investig. 2011;2:92–100. [PMC free article: PMC4015537] [PubMed: 24843467]
- 263.
- Hameed S, Jaffe A, Verge CF. Advances in the detection and management of cystic fibrosis related diabetes. Curr Opin Pediatr. 2015;27:525–533. [PubMed: 26087430]
- 264.
- Ren H, Qin L, Wang W, Ma J, Zhang W, Shen PY, Shi H, Li X, Chen N. Abnormal glucose metabolism and insulin sensitivity in Chinese patients with Gitelman syndrome. Am J Nephrol. 2013;37:152–157. [PubMed: 23392128]
- 265.
- Boyle P. Cushing's disease, glucocorticoid excess, glucocorticoid deficiency and diabetes. Diabetes Review. 1993:1.
- 266.
- Ganda OP. Growth hormone, acromegaly and diabetes. Diabetes Reviews. 1993:1.
- 267.
- Wass JA, Cudworth AG, Bottazzo GF, Woodrow JC, Besser GM. An assessment of glucose intolerance in acromegaly and its response to medical treatment. Clin Endocrinol (Oxf). 1980;12:53–59. [PubMed: 7379314]
- 268.
- Cryer P. Catecholamines, pheochromocytomas and diabetes. Diabetes Review. 1993:1.
- 269.
- Stenstrom G, Sjostrom L, Smith U. Diabetes mellitus in phaeochromocytoma. Fasting blood glucose levels before and after surgery in 60 patients with phaeochromocytoma. Acta Endocrinol (Copenh). 1984;106:511–515. [PubMed: 6475457]
- 270.
- Boden G. RJ CX. Glucagonoma syndrome, glucagon and glucose tolerance. Diabetes Review. 1993:1.
- 271.
- Vinik A, Feliberti E, Perry RR. Glucagonoma Syndrome. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth (MA)2000.
- 272.
- Andersen OO, Friis T, Ottesen B. Glucose tolerance and insulin secretion in hyperthyroidism. Acta Endocrinol (Copenh). 1977;84:576–587. [PubMed: 320805]
- 273.
- Repaske DR. Medication-induced diabetes mellitus. Pediatr Diabetes. 2016;17:392–397. [PubMed: 27492964]
- 274.
- Houston MC. The effects of antihypertensive drugs on glucose intolerance in hypertensive nondiabetics and diabetics. Am Heart J. 1988;115:640–656. [PubMed: 3278578]
- 275.
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–58. [PubMed: 9619798]
- 276.
- Johnson C, Ahsan N, Gonwa T, Halloran P, Stegall M, Hardy M, Metzger R, Shield C 3rd, Rocher L, Scandling J, Sorensen J, Mulloy L, Light J, Corwin C, Danovitch G, Wachs M, van Veldhuisen P, Salm K, Tolzman D, Fitzsimmons WE. Randomized trial of tacrolimus (Prograf) in combination with azathioprine or mycophenolate mofetil versus cyclosporine (Neoral) with mycophenolate mofetil after cadaveric kidney transplantation. Transplantation. 2000;69:834–841. [PubMed: 10755536]
- 277.
- First MR, Gerber DA, Hariharan S, Kaufman DB, Shapiro R. Posttransplant diabetes mellitus in kidney allograft recipients: incidence, risk factors, and management. Transplantation. 2002;73:379–386. [PubMed: 11884934]
- 278.
- Jarrett RJ. Why is insulin not a risk factor for coronary heart disease? Diabetologia. 1994;37:945–947. [PubMed: 7806026]
- 279.
- Reaven GM, Chen YD. Role of insulin in regulation of lipoprotein metabolism in diabetes. Diabetes Metab Rev. 1988;4:639–652. [PubMed: 3069396]
- 280.
- Helfgott SM. Stiff-man syndrome: from the bedside to the bench. Arthritis Rheum. 1999;42:1312–1320. [PubMed: 10403257]
- 281.
- Taylor SI. Lilly Lecture: molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes. 1992;41:1473–1490. [PubMed: 1327927]
- 282.
- Zhou P, Ten S, Sinha S, Ramchandani N, Vogiatzi M, Maclaren N. Insulin receptor autoimmunity and insulin resistance. J Pediatr Endocrinol Metab. 2008;21:369–375. [PubMed: 18556968]
- Review Etiology and Pathogenesis of Diabetes Mellitus IN Children.[Endotext. 2000]Review Etiology and Pathogenesis of Diabetes Mellitus IN Children.Ergun-Longmire B, Maclaren NK. Endotext. 2000
- Prevalence of β-cell antibodies and associated autoimmune diseases in children and adolescents with type 1 diabetes (T1DM) versus type 2 diabetes (T2DM) in Qatar.[Acta Biomed. 2018]Prevalence of β-cell antibodies and associated autoimmune diseases in children and adolescents with type 1 diabetes (T1DM) versus type 2 diabetes (T2DM) in Qatar.Alyafei F, Soliman A, Alkhalaf F, Sabt A, De Sanctis V, Elsayed N, Waseef R. Acta Biomed. 2018 May 23; 89(S5):32-39. Epub 2018 May 23.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Hypoglycemia During Therapy of Diabetes.[Endotext. 2000]Review Hypoglycemia During Therapy of Diabetes.Davis HA, Spanakis EK, Cryer PE, Siamashvili M, Davis SN. Endotext. 2000
- Review Nonalcoholic Fatty Liver Disease: The Overlooked Complication of Type 2 Diabetes.[Endotext. 2000]Review Nonalcoholic Fatty Liver Disease: The Overlooked Complication of Type 2 Diabetes.Akshintala D, Chugh R, Amer F, Cusi K. Endotext. 2000
- Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents - End...Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...