Abstract
Identifying distinctions between pathogenic HIV and simian immunodeficiency virus (SIV) infections and nonprogressive SIV in natural African primate hosts might provide key insights into HIV pathogenesis. Similar to pathogenic HIV infection in humans, natural SIV infections result in high viral replication and massive acute depletion of mucosal CD4+ T-cells. A key distinction of natural SIV infections is a rapidly-developing anti-inflammatory milieu that prevents chronic activation, apoptosis and proliferation of T-cells and preserves the function of other immune cell subsets, thus contributing to the integrity of the mucosal barrier and the lack of microbial translocation from the gut to the peritoneum. Immunologic features observed during natural SIV infections suggest approaches for designing new strategies for producing novel second generation vaccines and therapeutic approaches to inhibit disease progression in HIV-infected humans.
Introduction
Twenty-five years after its discovery, the correlates of immune protection against human immunodeficiency virus (HIV) are still not known and an effective vaccine is not yet available. Moreover, recent results from clinical vaccine trials are disappointing [1] and it is clear that a better understanding of pathogenesis is necessary to devise new strategies for HIV infection control [1]. One area of investigation that offers promise for uncovering new insights to contain HIV-induced immunopathogenesis is the assessment of natural SIV infections of African nonhuman primates (NHPs). Once considered exotic models of infection of distant monkey species with divergent virus strains, recent developments now indicate that these same animal models might hold essential clues for reconsidering and expanding the current paradigms of HIV pathogenesis.
To date, more than 40 primate lentiviruses have been found to naturally infect African NHP hosts at high prevalence levels [2]. In general, SIVs infecting African species evolved through host-dependent evolution, meaning that they have infected different NHP species for thousands, or hundreds of thousands of years, possibly since primate speciation [2]. During this long history, SIVs and their natural hosts evolved to coexistence, with the African NHPs infected with their species-specific viruses generally not showing signs of SIV-induced disease or simian AIDS [2,3]. However, a handful of AIDS cases described recently in African NHPs indicate that the lack of disease progression in natural hosts is not due to infection with unharmful viruses, but to an effective control of disease progression [4–6]. This control of disease progression is not necessarily due to a robust and effective immune response but rather to a better management of the deleterious consequences of lentiviral infection, such as an effective control of the aberrant immune activation (see below). Furthermore, upon cross-species transmission, SIVs that naturally infect African NHPs show a spectacular increase in pathogenicity: HIV-1 and HIV-2 have been shown to have a simian origin [2,7,8], resulting from independent cross-species transmissions of SIVs. Thus, HIV-1 originated from cross-species transmission of two distinct viruses; SIVcpz (the virus naturally infecting chimpanzees), which is at the origin of HIV-1 groups M and N [7], and SIVgor (the virus naturally infecting gorillas), which is at the origin of HIV-1 group O [8]. Similarly, HIV-2 resulted from multiple cross-species transmissions of SIVsmm, the virus that naturally infects sooty mangabeys (SMs) [2]. Although both HIV-1 and HIV-2 are pathogenic in humans, their simian ancestors infect their natural hosts (chimpanzees, gorillas and SMs) without major clinical consequences [2,9,10]. Similarly, experimental or accidental cross-species transmission of several SIVs from different African NHP species to Asian macaques resulted in disease progression [11–14]. Unraveling the mechanism of the control of disease progression in natural hosts of SIV is essential to discern why there is an increase in pathogenicity upon cross-species transmission of the SIVs to humans or macaques.
Despite the large number of lentiviruses that infect African NHPs, knowledge of the pathogenesis of natural SIV infections, as well as after cross-species transmission, is derived from only three animal models: African green monkeys (AGMs), SMs and mandrills. There are several reasons for this: (i) most lentiviruses infecting natural hosts are only known from sequences and have never been isolated, fully characterized and inoculated into natural or heterologous hosts [2]; (ii) numerous African NHPs are not available in research facilities or accessible through importation; (iii) the majority of African NHPs are highly endangered, therefore SIV pathogenesis studies are not possible in these species. An animal model of non-progressive infection circumventing both difficulties of importation from Africa and problems related to the endangered nature of most African species have been recently developed by infecting Caribbean AGMs with SIVagm.sab, the virus naturally infecting sabaeus AGMs in West Africa [15]. Using the available models, common features (Box 1) as well as significant differences (Box 2) have been identified between pathogenic and non-progressive SIV infections.
Box 1. Similarities between nonprogressive SIV infections in natural hosts and pathogenic HIV and SIV infections.
Similar high levels of viral replication during the primary infection
Similar degree of CD4+ T-cell depletion in blood and intestine during the acute infection indicative that acute loss of CD4+ T-cells is not the main immunopathogenic factor driving progression to AIDS
Identical target cells for SIVagm and SIVmac during primary and chronic infection, resulting in similar dynamics of effector memory CD4+ T-cells
Similar in vivo biology of the viruses infecting progressive and nonprogressive hosts. Similar half-life duration for the viruses and infected cells in progressive versus nonprogressive models
Similar rates of mutation of the SIVs during progressive and non-progressive infections, most likely resulting as a consequence of similar host selective pressures and same high levels of viral replication
Similar limited role of humoral immune responses in controlling chronic viral replication
Cellular immune responses of the same magnitude between non-progressive and progressive hosts
Similar levels of virus in the central spinal fluid (CSF)
Box 2. Differences between nonprogressive SIV infections in natural hosts and pathogenic HIV and SIV infections.
Lack of disease progression in the vast majority of cases of natural SIV infections. Progression to AIDS in the vast majority of pathogenic infections
Lower levels of CD4+ T-cells and CCR5+ CD4+ T-cells in natural hosts of SIVs. High levels of CD4+ T-cells and CCR5-expressing CD4+ T-cells in hosts that progress to AIDS
Remarkably stable viral replication (for decades) during chronic infection is in natural SIV infections; significant increases with disease progression in pathogenic HIV and SIV infections
Restoration or stabilization of CD4+ T-cells in periphery and intestine during the chronic phase in nonprogressive infections; transient partial restoration of mucosal CD4+ T-cells in HIV-1 infection; no significant restoration in highly pathogenic SIVmac infection of macaques
Massive chronic CD4+ T-cell depletion in subsets of SIV-infected non-progressive hosts does not necessarily result in disease progression
Preservation of functionality of various immune cell subsets (including; CD4+, CD8+, γδ T-cells) in natural SIV hosts
Coreceptor expansion may occur in natural infections, but it is not associated with changes in the rates of disease progression. Some SIVs naturally infecting African nonhuman primate hosts are dual CCR5 and CXCR4-tropic without evidence of onset of clinical signs of AIDS
Normal levels of immune activation, apoptosis and cell proliferation during chronic infection of natural hosts. These factors may be the reason for CD4 restoration in the context of high viral replication
Lack of SIV enteropathy and maintenance of function and numbers of other immune cell subsets in natural infections. Major potential consequence being the lack of microbial translocation (pre-infection levels of LPS), which might explain the lack of aberrant immune activation during chronic SIV infection in natural hosts
Establishment of a rapid anti-inflammatory milieu in natural hosts that prevents chronic aberrant immune activation
Differences in the baseline levels and in the dynamics of Tregs
Lower levels of antibodies both quantitatively and qualitatively (i.e., absence of anti-Gag antibodies) in natural infections
Lack of vertical transmission in natural SIV hosts
No sign of neurological disease in non-progressive infections of natural hosts
General characteristics of natural SIV infections
The paradox of natural SIV infections: high viral replication but preservation of peripheral CD4+ T-cells
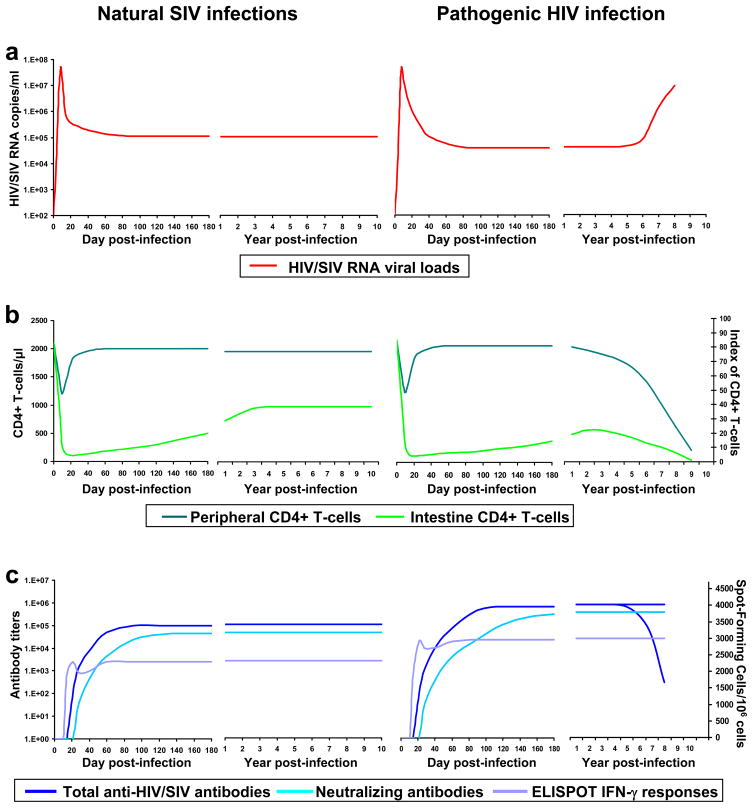
All studies carried out thus far reported high levels of SIV replication in natural African primate hosts during both acute and chronic infection [14,16–29] (Figure 1a). During primary infection, these levels of viral replication are similar to those observed in pathogenic HIV-1 and SIVmac infections of humans and macaques [14,18,19,21–23,25–28,30,31] (Figure 1a). During chronic infection, the levels of SIV replication in natural hosts are slightly higher than those observed in HIV-1-infected individuals or SIV-infected macaques with normal progression to AIDS (Figure 1a) [26]. Over the chronic infection, viral replication is remarkably steady in natural hosts [16,24,25] while, in contrast, it shows significant variation and increases with disease progression in pathogenic infections (Figure 1a) [2]. In conclusion, the ability of natural hosts to resist disease progression does not reside in low levels of SIV replication.
Figure 1. Comparison of major parameters of SIV infections in natural hosts and HIV-1 infection in humans.
a. The levels of viral replication are similar between naturally-infected non-human primates (NHP) and HIV-1-infected patients. The major difference occurs in the late stage of the chronic infection, when the steady state is maintained in natural SIV infection but viral loads increase in HIV-1-infected patients with consequent disease progression. b. The dynamics of peripheral CD4+ T-cells, as well as mucosal (intestinal) CD4+ T-cells is generally similar during acute infection in both natural and pathogenic types of infection. During the chronic phase (right hand panels in each group), there is a partial restoration in naturally infected group resulting in the stabilization of the mucosal CD4+ T-cell pool, while in pathogenic HIV infection, a partial restoration occurs only in the initial stages, and CD4+ T-cell depletion continues to exhaustion with disease progression. c. There are no significant differences in humoral immune responses (illustrated here by total anti-HIV or SIV antibody and neutralizing antibody titers) or cellular immune responses (measured by IFN-γ ELISPOT). Both humoral and cellular immune responses are slightly lower in natural infections compared to pathogenic HIV-1 infection. Plots are synthesized from papers published both by ourselves and others and indicate average values.
Interestingly, in the context of these high levels of viral replication peripheral CD4+ T-cells are generally well preserved during chronic SIV infection in natural hosts [16–19,21–24,30,32,33]. While CD4+ T-cell preservation occurs in the majority of natural hosts, analysis of the SIV-infected sooty mangabeys has determined that CD4+ T-cell depletion can occur sometimes to AIDS defining levels with no apparent clinical consequences [16,34,35]. Therefore, although some exceptions do exist, the general preservation of CD4+ T-cells in naturally-infected African hosts is one of the major differences with pathogenic HIV-1 and SIVmac infections, in which the level of peripheral CD4+ T-cells is one of the parameters defining disease progression [36] (Figure 1b).
In addition, African species that are natural hosts of SIVs have very low expression of the viral entry receptor CCR5 on CD4+ T-cells, especially in the intestine [37], which is the main site of viral replication and CD4+ T-cell depletion in pathogenic SIVmac or HIV-1 infection [38–42]. This low expression of CCR5 is specific for the CD4+ T-cell subset, as CCR5 expression of CD8+ T-cells is in the same range as for progressive hosts (humans or macaques) [37]. In stark contrast, progressive hosts show high percentages of CD4+ T-cells expressing CCR5 at the mucosal sites [37]. CCR5 being the main co-receptor of HIV and SIV, its reduced expression on CD4+ T-cells suggested a low availability of target cells, initially offering an explanation for the preservation of the CD4+ T-cells in SIV-infected natural hosts, as a consequence of reduced CD4+ T-cell susceptibility.
In addition to low CCR5 expression on CD4+ T-cells, some African NHP species that are natural hosts of SIVs, such as the AGMs, also harbor low levels of CD4+ T-cells, especially in the intestine, even in the absence of SIV infection [15]. Both lower levels of CD4+ and CD4+CCR5+ T-cells may be the result of co-evolution of African NHPs with their species-specific SIVs [37]. Thus, the low levels of CCR5+ CD4+ target cells in natural infections, may be involved in preventing virus transmission, as recently reported in mandrills, where low levels of CCR5+ CD4+ T-cells in offspring were associated with lack of breast-feeding transmission [43].
The paradox of paucity of CD4+CCR5+ target cells, good preservation of peripheral CD4+ T-cells (Figure 1b) and high levels of viral replication raises several important questions regarding the pathogenesis of SIV infection in natural hosts: (i) Are immune responses involved in the control of viral replication and infection outcome? (ii) Will the low CCR5 expression make the mucosal CD4+ T-cells less affected by SIV infection? (iii) Is there a preservation of CD4+ T-cells in the intestine of natural hosts? (iv) Would this preservation of mucosal CD4+ T-cells be responsible for lack of disease progression in natural hosts? (v) If CD4+ T lymphocytes are not responsible for the high levels of SIV infection, what cellular subset is accountable for the high viral replication in the natural host?
Immune responses in natural SIV infections are not enhanced compared to those observed in pathogenic infections
As expected from the high levels of viral replication, robust immune control is not the cause of the lack of pathogenicity of natural SIV infections. The level of SIV-specific T-cell responses in SMs and AGMs is not stronger or broader than those observed in pathogenic SIV or HIV infections of macaques or humans (Figure 1c) [44,45]. Note, that one cannot discount a role for SIV specific cytotoxic T lymphocytes (CTL) in controlling post-acute phase viral replication from increasing still further than is observed. However, the impact of cellular immune responses on virus control is not significantly different from that observed in pathogenic infections.
Studies regarding humoral immune responses indicate that anti-SIV specific antibodies are indeed present following the post-acute phase of the infection [21–23,34] and humoral immune responses are similar or lower in natural hosts compared to pathogenic infection (Figure 1c) [2,14,46]. It is thus likely that B cells do not play a significant role in controlling SIV’
Therefore, the lack of disease progression in natural SIV hosts appears to not rely on robust immune responses, an observation that has tremendous implications for HIV and SIV vaccines designed to suppress virus replication levels as an endpoint goal [3].
SIV infects the same target cells and induces similar massive mucosal CD4+ T-cell depletion in natural nonprogressive and in pathogenic infections
Studies in pathogenic HIV-1 and SIVmac infections concluded that disease progression is predicted by a massive loss of CD4+ T-cells at mucosal sites during the primary infection [38–41,47]. This conclusion has sobering implications for HIV-infected patients, as it implies that a lack of intervention during the very early phase of lentivirus infection (a task virtually impossible to accomplish due to the delay in HIV diagnosis) reduces the chances of a successful therapeutic intervention at later stages of the infection.
However, our studies in natural hosts reported a massive mucosal CD4+ T-cell depletion during the acute SIV infection, of the same magnitude as that reported in pathogenic infections (Figure 1b) [19,27]. The finding that an acute CD4+ T-cell depletion in the intestine is a common feature for all types of SIV infection: progressive (in both rapid progressors and normal progressors), persistent nonprogressive and completely controlled [19,27] suggests that acute mucosal CD4+ T-cell depletion is not predictive for disease progression in NHPs.
During acute infection of natural hosts, SIVs replicate predominantly in lymphocytes, as demonstrated by the massive depletion of CD4+ T-cells in the gut and by SIV colocalization with CD3+ T-cells, but only infrequently in macrophages [25,27,34,48]. Similar to pathogenic infections, effector memory CD4+ T-cells are preferentially depleted in the intestine during acute natural SIV infections, with central memory and naïve CD4+ T-cells being affected as well [19,27]. Furthermore, again similar to pathogenic infections, effector memory CD4+ T cells remain depleted during chronic SIV infection of the natural host, the recovery being sustained mainly on the central memory and naïve cells [27]. SIVagm-infected cells are negative for the proliferation marker Ki-67 [27], as also reported for pathogenic SIVmac [38]. Therefore, these recent findings demonstrate that the lack of AIDS progression in natural SIV hosts is not due to preservation of key mucosal CD4+ T-cell subpopulations and show that it is possible to survive SIV infection with a highly replicative virus that can kill the majority of the CD4+ T-cells in the intestine during acute infection. Nonprogression in the natural host models therefore appears to be due to host mechanisms that control the deleterious consequences of SIV infection during the chronic phase of the SIV infection.
Thus, investigations in natural hosts have challenged one of the core paradigms in HIV pathogenesis by demonstrating that: (i) Intestinal CD4+ T-cells are depleted in natural hosts despite their low level of CCR5; (ii) The lack of disease progression in African species is not due to maintenance of CD4+ T-cells in the intestine during the primary infection; (iii) There is no threshold of mucosal CD4+ T-cell depletion predicting progression to AIDS and that intestinal CD4+ T cell depletion is unlikely to be useful as a prognostic for disease progression in pathogenic infections.
There is, however, a major difference between pathogenic infections of humans and macaques and nonprogressive infections of natural hosts: during the chronic infection of AGMs, there is a significant, albeit partial, restoration of mucosal CD4+ T-cells in the natural hosts (Figure 1b) [27]. In chronically infected SMs, this restoration is evident in some individuals, but generally the mucosal CD4+ T-cell levels remain stable during chronic infection [19]. Mucosal CD4+ T-cell restoration is somewhat surprising, as it occurs in the context of the high levels of viral replication. In so-called elite controlled infections of humans and macaques, this restoration is also observed, but occurs in the context of control of viral replication [27,49]. This observation raises additional questions on the pathogenic mechanisms leading to nonprogression: (i) Is the high plasma viremia in the natural host a true reflection of a high level of an infective virus? and (ii) Are other cellular subsets (such as the macrophages) responsible for the high viral replication in the natural host, at least during the chronic infection?
The answer to the first question is relatively simple, as the rates of non-synonymous mutations of the SIV infecting natural hosts are similar to those reported for pathogenic HIV-1 and SIVmac infections indicating high levels of selective pressures [50–52]. High rates of non-synonymous mutations also provide evidence that the high levels of viral particles identified in the plasma are derived from productively infected cells and constantly mutating to avoid the host immune response. The second question has received a lot of attention recently, especially in the context of the observation of the paucity of CCR5 target cells discussed above. There are multiple lines of evidence against a substantial role for macrophages as a major source of virus replication in natural infections: (i) The dynamics of virus replication during acute infection is strikingly similar between progressive and nonprogressive SIV infections [26], which likely would not be the case if SIV replication in natural hosts is supported by long-lived infected cells that produce virus slowly; (ii) The massive acute depletion of mucosal CD4+ T-cells suggests that these cells are the SIV targets [19,27]. In addition, experimental depletion with a specific antibody that targets CD4+ T-cells and not macrophages results in a significant decrease in plasma viral loads in SMs [53]. In contrast, depletion of macrophages in SIVsmm-infected SMs did not reduce viral loads [53]. Furthermore, in a subset of SMs showing significant CD4+ T-cell depletion during the course of chronic infection, the levels of plasma viral loads were also dramatically reduced [16,34]. (iii) Direct evidence by in situ hybridization that during acute infection of AGMs, SIVagm replicates in lymphocytes and not in macrophages [25,27]; (iv) Finally, experimental administration of antiretroviral treatments to AGMs and SMs chronically infected with SIV clearly showed that the bulk of SIV replication occurs in short-lived cells [25,48] (with average an in vivo lifespan shorter than that estimated in HIV-1-infected humans and SIVmac-infected macaques) [54]. Collectively these data show that, in natural hosts, SIVs preferentially infect short-lived cells (such as lymphocytes) during both acute and chronic infection.
What makes the natural hosts of SIVs resistant to disease progression?
Lack of aberrant levels of immune activation, cell proliferation and apoptosis protect natural hosts from the deleterious consequences of SIV infection
A large and growing body of evidence suggests that the establishment of a state of chronic, generalized immune activation is a major determinant of disease progression in pathogenic HIV-1 and SIVmac infections [55–61]. Some of the best data supporting the immune activation hypothesis is derived from studies of natural hosts of SIV infection, as these monkeys routinely maintain low levels of immune activation that prevent chronic immune exhaustion and progression to AIDS. Although the precise extent of immune activation in HIV infection is yet to be fully defined, there are a number of ways to measure immune activation in infected patients including: (i) The frequency of T-cells expressing markers of activation and proliferation; (ii) The levels of activation-induced apoptosis of uninfected T-cells; (iii) The levels of T-cell proliferation as measured by cellular labeling; and (iv) The levels of pro-inflammatory cytokines in plasma [55]. These analyses performed in SIV-infected natural hosts during acute and chronic infection have confirmed consistent distinctions in the dynamics of immune activation between SIV infection in natural hosts and pathogenic HIV-1 or SIVmac infections [14,18,19,22–25,27,28,32–34,62]. Testing the dynamics of immune activation markers (HLA-DR and CD69) demonstrated transient and low increases on T-cells (CD8+ T-cells predominantly) which only occurs during acute infection (Figure 2a) [14,18,19,22–25,27,28,32–34,62]. During chronic infection, immune activation markers are similar to, or slightly elevated from, preinfection levels in SIV-infected African hosts, in contrast to pathogenic HIV-1 and pathogenic SIVmac infections, in which immune activation levels correlate with disease progression [55]. Low levels of immune activation were correlated with low levels of apoptosis in SIV-infected natural hosts, which does not significantly increase during either acute [27,63] or chronic [32,64] SIV infection in natural hosts (Figure 2a). This result also contrasts HIV-1 and SIVmac infections, where apoptosis is one of the mechanisms responsible for the massive loss of CD4+ T-cells despite a relatively low number of virally-infected cells [38,64]. Also in contrast to progressive SIVmac and HIV-1 infections, where an excessive cell turnover was reported to be a result of the massive CD4+ T-cell loss and the consequent attempts by the host immune system to replace this cell loss [65,66], T-cell proliferation was increased in the natural hosts only during primary infection (Figure 2) [19,27,28,34,67]. During chronic SIV infection of natural hosts proliferation levels returned to preinfection levels or remained slightly above background levels [67–69]. Finally, the levels of pro-inflammatory cytokines such as IFN-γ, TNF-α or IL-12 were normal during the infection in the natural hosts compared to significantly elevated levels in SIVmac-infected macaques (Figure 2b) [33,34,62]. This differences are significant, as it was recently shown that HIV proteins target TNF receptor signaling, leading both to apoptosis of uninfected bystander T-cells and to sustained viral replication in infected T-cells and macrophages, that can explain both immune suppression and the formation of viral reservoirs during HIV infection [70].
Figure 2. Major differences observed between non-pathogenic natural SIV infections and pathogenic SIVmac infection of rhesus macaques, prevent disease progression in natural hosts and result in disease progression in SIVmac-infected macaques.
An anti-inflammatory milieu (illustrated here by the dynamics of IL-10 and Fox-P3, a T-regulatory cell marker) is rapidly installed in natural hosts after SIV infection (starting from day 1 post-infection). As a result, the levels of immune activation (illustrated here by the levels of HLA–DR expression on both CD4+ and CD8+ T-cells), T-cell proliferation (illustrated by the levels of Ki-67+ CD8+ T-cells) and apoptosis of CD4+ T-cells in the intestine are relatively well controlled in natural infections (a). Pro-inflammatory cytokines (illustrated by the dynamics of IFN-γ in plasma) are only transiently increased at low levels (b). As a consequence, the integrity of the mucosal immunological barrier is maintained (as illustrated by the dynamics of plasma LPS levels) (c), which in turn prevents chronic immune activation and disease progression. In pathogenic SIVmac infection of rhesus macaques, a pro-inflammatory milieu results in excessive immune activation, apoptosis and T-cell proliferation, generating severe damage of the mucosal barrier and microbial translocation which maintains the chronic immune activation and results in disease progression. Cellular markers are presented as percentages of the major T-cell population. Soluble read-outs such as cytokines (ng/ml) and LPS (pg/ml) are presented as plasma concentrations. Fox-P3 gene expression is shown as the fold increase within peripheral blood monocytes (PBMCs). Plots summarize data from previously published papers from ourselves and others and represent average values.
Normal levels of immune activation in the natural hosts are probably involved in the maintenance of numbers and of the normal function of a variety of immune cell subsets [71–73], in contrast to progressive infections in which disturbances of regulatory T cells (Tregs), Th17, natural killer cells, monocytes, and γδ T-cells were described [65,74]. All these cells ensure that the natural hosts maintain their defenses against microorganisms despite the T helper loss in the intestine and protect them from developing the opportunistic infections and cancers seen in progressive hosts in which a general loss of number and function of all these immune cells occurs.
Together, these findings point to a major difference between pathogenic and nonpathogenic SIV infections which may be responsible for both the lack of SIV enteropathy in the natural hosts and also the recovery of the CD4+ T-cells in the presence of high levels of viral replication in nonprogressive infections. Both of these aspects are essential in preventing disease progression in natural infections. Therefore, understanding the mechanisms contributing to the lack of immune activation during natural SIV infections has become one of the highest priorities in this field.
Mechanisms underlying the lack of immune activation during SIV infection in natural hosts
Recent studies in the macaque model showed that the major depletion of CD4+ T-cells in the gut is accompanied by increased levels of apoptosis of the intestinal epithelia and by heavy inflammatory infiltrates in the lamina propria that together define SIV enteropathy [38,74]. The bacterial translocation hypothesis first proposed by Douek and Brenchley states that an altered intestinal barrier results in release of commensal flora components into the circulation of both SIVmac-infected macaques and HIV-1 infected humans [74,75]. It was proposed that these microbial products released from a damaged mucosal barrier bind to toll-like receptors (TLRs), thus driving the continuous and chronic stimulation of the immune system observed during pathogenic SIVmac and HIV-1 infections [74,75]. The chronic aberrant immune activation characteristic of these pathogenic infections finally results in a functional failure of numerous immune cell subsets and exhaustion of the regenerative capacities of the host, contributing to disease progression.
Natural hosts avoid SIV enteropathy despite the massive CD4+ T-cell loss and high viral replication during primary infection. In SIV-infected AGMs, the mechanism of enteropathy prevention relies in their ability to mount an early anti-inflammatory response that likely involves Tregs [33]. A rapid increase in the anti-inflammatory cytokine TGF-β was reported to be involved in early generation of Tregs, as shown by significant increases in Fox-P3 expression in SIVagm-infected AGM PBMCs which was described to occur as early as 24 hours post infection (Figure 2b), accompanied by increases in the levels of the anti-inflammatory (IL-10) cytokines (Figure 2b) [33]. This Treg generation response is delayed in SIVmac-infected macaques, and is therefore likely to be inefficient (Figure 2b) [33]. The anti-inflammatory milieu engendered very early in SIV-infected natural hosts might explain the lack of enteropathy and corroborate the normal levels of plasma lipopolysaccharide (LPS) observed during both acute and chronic natural SIV infections (Figure 2c) and be indicative of an intact intestinal barrier [19,27]. Maintenance of gut mucosal integrity precludes the release of bacterial microfloral TLR ligands and thus the chronic immune stimulation and progression to AIDS.
In pathogenic infections, other immune subsets become dysfunctional during HIV infection including CD8+ T-cells, B cells, macrophages, natural killer cells, or γδ T-cells [65,74]. γδ T-cells are particularly interesting as they play an important role in the recognition of microbial pathogens in the gut and can influence adaptive immune responses [76]. HIV infection results in the induction of γδ T-cell anergy [77] and the inability of γδ T-cells to migrate in response to pro-inflammatory chemokines [78]. In contrast, γδ T-cells from SIV-infected SMs maintain function due to their ability to proliferate in response to the bacterial antigens [34], and express Th1 cytokines in response to γδ T-cell specific and non-specific stimuli [71]. It is likely that the ability of SIV-infected natural hosts to maintain an intact mucosal integrity and remain free of simian AIDS is also due to an intact functionality of these multiple immune cell subsets.
In addition, viral proteins of some SIV naturally-infecting African NHP hosts might contribute to the low levels of immune activation. Indeed, nef alleles from primate lentiviruses, including HIV-2, down-modulate TCR-CD3 from infected T-cells, therefore possibly blocking their responsiveness to activation [79]. Thus, Nef-mediated suppression of T-cell activation might be a fundamental property of primate lentiviruses that likely evolved to maintain viral persistence in the context of an intact host immune system [79].
Concluding remarks: assembling a new paradigm of HIV pathogenesis
We have described here the key aspects of SIV infection in natural hosts that have implications with regard to some core paradigms of HIV pathogenesis (Figure 3). For example, acute mucosal CD4+ T-cell depletion is a common feature of lentiviral infection (pathogenic, persistent non-progressive, and controlled) and therefore is not predictive for disease progression. However, important distinctions regarding the stabilization or restoration of the intestinal mucosa have been observed during chronic lentiviral infection of the SIV infected natural hosts. Stabilization or restoration occurs in the context of active viral replication, but normal levels of immune activation, T-cell proliferation and apoptosis. Lack of aberrant immune activation maintains functionality of other multiple immune cell subsets which, in turn, may also contribute to the lack of disease progression. All these characteristics result in an active persistent infection, but generally no disease progression in the natural hosts of SIV. This observation has tremendous impact on clinical approaches aimed at controlling disease progression in HIV-1-infected patients, identifying immune-based therapies as a viable approach for controlling disease progression.
Figure 3. Comparison of events associated with natural SIV infection and pathogenic SIV and HIV-1 infection.
Differences in host response to infection are responsible for the outcome of the infection: a balanced host response in natural infection (left hand side) shuts down most of the factors that have deleterious consequences for the infected hosts (immune activation, apoptosis, excessive inflammation, enteropathy). In pathogenic infections, excessive host responses result in mucosal damage and consequent translocation of gut microbial products, immune system exhaustion and progression to AIDS (right hand side). Red boxes indicate deleterious events whereas green boxes beneficial ones.
Finally, the observation that a balanced response to the virus might prevent disease progression in natural SIV infections while not suppressing viral replication, should impact vaccination strategies currently aimed at stimulation of adaptive immune responses. As adaptive immunity provides only partial control of virus replication in natural hosts, similar to pathogenic HIV or SIV infections, it is unlikely that focusing only on approaches that stimulate HIV-specific cellular immune responses or antibodies will result in a reduced pathogenic outcome in HIV-infected patients. We conclude that stimulation of innate immune responses and suppression of systemic immune activation will be key elements in preventing HIV disease progression, similar to natural SIV hosts. Further studies in SIV infection in natural hosts could generate new strategies that are required to develop the second generation vaccine and immune-based therapies designed to inhibit immune activation that might be beneficial for preventing HIV-1-infected patients from progressing to AIDS.
Acknowledgments
We would like to thank Françoise Barre-Sinoussi, Beatrice H. Hahn, Satya Dandekar, Vanessa M. Hirsch, Amitinder Kaur, Michaela Muller-Trutwin, Daniel Douek, Andrew A. Lackner, Preston A. Marx, Louis Picker, Sentob Saragosti, François Simon for sharing unpublished information and for helpful discussions of data and hypotheses. This work was supported by NIH/NIAID/NCRR grants RO1 AI064066 and R21AI069935 (IP), R01 AI035522 and R21 AI060451 (DLS), RO1 AI066998 (GS), R01 AI065325 and P20 RR020159 (CA), and RR-00168 (TNPRC) RR-00165 (YNPRC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205 (1):7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VandeWoude S, Apetrei C. Going wild: Lessons from T-lymphotropic naturally occurring lentiviruses. Clin Microbiol Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvestri G, et al. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117 (11):3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apetrei C, et al. Direct inoculation of simian immunodeficiency virus from sooty mangabeys in black mangabeys (Lophocebus aterrimus): first evidence of AIDS in a heterologous African species and different pathologic outcomes of experimental infection. J Virol. 2004;78 (21):11506–11518. doi: 10.1128/JVI.78.21.11506-11518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling B, et al. Classic AIDS in a sooty mangabey after an 18-year natural infection. J Virol. 2004;78 (16):8902–8908. doi: 10.1128/JVI.78.16.8902-8908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandrea I, et al. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS. 2001;15 (18):2461–2462. doi: 10.1097/00002030-200112070-00019. [DOI] [PubMed] [Google Scholar]
- 7.Keele BF, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313 (5786):523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Heuverswyn F, et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444 (7116):164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S, et al. The call of the wild: What can be learned from studies of SIV infection of natural hosts? In: Leitner T, et al., editors. HIV Sequence Compendium 2004. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; NM: 2005. pp. 2–29. [Google Scholar]
- 10.Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol. 2005;34 (5–6):243–252. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 11.Apetrei C, et al. Molecular epidemiology of SIVsm in US Primate Centers unravels the origin of SIVmac and SIVstm. J Virol. 2005;79 (14):8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apetrei C, et al. Kuru experiments triggered the emergence of pathogenic SIVmac. AIDS. 2006;20(3):317–321. doi: 10.1097/01.aids.0000206498.71041.0e. [DOI] [PubMed] [Google Scholar]
- 13.Beer BE, et al. Immunodeficiency in the absence of high viral load in pigtailed macaques infected with simian immunodeficiency virus SIVsun and SIVlhoest. J Virol. 2005;79 (22):14044–14056. doi: 10.1128/JVI.79.22.14044-14056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souquière S, et al. SIVmnd-1 and SIVmnd-2 have different pathogenic potentials in Rhesus macaques upon experimental cross-species transmission. 2008 doi: 10.1099/vir.0.005181-0. submitted. [DOI] [PubMed] [Google Scholar]
- 15.Pandrea I, et al. Simian immunodeficiency virus (SIV) SIVagm. sab infection of Caribbean African green monkeys: New model of the study of SIV pathogenesis in natural hosts. J Virol. 2006;80 (10):4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apetrei C, et al. Virus subtype-specific features of natural simian immunodeficiency virus SIVsmm infection in sooty mangabeys. J Virol. 2007;81 (15):7913–7923. doi: 10.1128/JVI.00281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broussard SR, et al. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol. 2001;75 (5):2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diop OM, et al. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J Virol. 2000;74 (16):7538–7547. doi: 10.1128/jvi.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon SN, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179 (5):3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gueye A, et al. Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. J Med Primatol. 2004;33 (2):83–97. doi: 10.1111/j.1600-0684.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- 21.Onanga R, et al. High levels of viral replication contrast with only transient changes in CD4+ and CD8+ cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J Virol. 2002;76 (20):10256–10263. doi: 10.1128/JVI.76.20.10256-10263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onanga R, et al. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx) J Virol. 2006;80 (7):3301–3309. doi: 10.1128/JVI.80.7.3301-3309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandrea I, et al. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J Virol. 2006;80 (10):4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandrea I, et al. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology. 2003;317 (1):119–127. doi: 10.1016/j.virol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Pandrea I, et al. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J Virol. 2008;82 (7):3713–3724. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandrea I, et al. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J Med Primatol. 2006;35 (4–5):194–201. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 27.Pandrea IV, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179 (5):3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestri G, et al. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005;79 (7):4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein S, et al. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J Virol. 2000;74 (24):11744–11753. doi: 10.1128/jvi.74.24.11744-11753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandrea I, et al. Impact of viral factors on very early in vivo replication profiles in simian immunodeficiency virus SIVagm-infected African green monkeys. J Virol. 2005;79 (10):6249–6259. doi: 10.1128/JVI.79.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein S, et al. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J Virol. 2006;80 (10):4868–4877. doi: 10.1128/JVI.80.10.4868-4877.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvestri G, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18 (3):441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 33.Kornfeld C, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115 (4):1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milush JM, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol. 2007;179 (5):3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 35.Sumpter B, et al. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol. 2007;178 (3):1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296 (12):1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 37.Pandrea I, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109 (3):1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434 (7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 39.Mattapallil JJ, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434 (7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 40.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200 (6):761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehandru S, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. 2007;81 (2):599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280 (5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 43.Pandrea I, et al. Paucity of CD4+CCR5+ T-cells may prevent breastfeeding transmission of SIV in natural non-human primate hosts. J Virol. 2008;82(11):5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunham R, et al. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108 (1):209–217. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, et al. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J Virol. 2006;80 (6):2771–2783. doi: 10.1128/JVI.80.6.2771-2783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirsch VM. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 2004;6 (1):40–53. [PubMed] [Google Scholar]
- 47.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200 (6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon SN, et al. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol. 2008;82 (7):3725–3735. doi: 10.1128/JVI.02408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saez-Cirion A, et al. HIV controllers: how do they tame the virus? Trends Immunol. 2007;28 (12):532–540. doi: 10.1016/j.it.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Vanderford TH, et al. Adaptation of a diverse simian immunodeficiency virus population to a new host is revealed through a systematic approach to identify amino acid sites under selection. Mol Biol Evol. 2007;24 (3):660–669. doi: 10.1093/molbev/msl194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller MC, Barre-Sinoussi F. SIVagm: genetic and biological features associated with replication. Front Biosci. 2003;8:d1170–1185. doi: 10.2741/1130. [DOI] [PubMed] [Google Scholar]
- 52.Demma LJ, et al. Evolution of the uniquely adaptable lentiviral envelope in a natural reservoir host. Retrovirology. 2006;3:19. doi: 10.1186/1742-4690-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klatt NR, et al. Availability of activated CD4+ T-cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118 doi: 10.1172/JCI33814. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perelson AS. Modelling viral and immune system dynamics. Nat Rev Immunol. 2002;2 (1):28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- 55.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22 (4):439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 56.Kovacs JA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194 (12):1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohri H, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194 (9):1277–1287. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17 (13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 59.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179 (4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 60.Sousa AE, et al. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169 (6):3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 61.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104 (4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 62.Ploquin MJ, et al. Distinct expression profiles of TGF-beta1 signaling mediators in pathogenic SIVmac and non-pathogenic SIVagm infections. Retrovirology. 2006;3:37. doi: 10.1186/1742-4690-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cumont MC, et al. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Virol. 2008;82 (3):1175–1184. doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurtrel B, et al. Apoptosis in SIV infection. Cell Death Differ. 2005;12(Suppl 1):979–990. doi: 10.1038/sj.cdd.4401600. [DOI] [PubMed] [Google Scholar]
- 65.Grossman Z, et al. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12 (3):289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 66.Picker LJ, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200 (10):1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muthukumar A, et al. Timely triggering of homeostatic mechanisms involved in the regulation of T-cell levels in SIVsm-infected sooty mangabeys. Blood. 2005;106 (12):3839–3845. doi: 10.1182/blood-2005-01-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakrabarti LA, et al. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol. 2000;74 (3):1209–1223. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur A, et al. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J Virol. 2008;82 (3):1084–1093. doi: 10.1128/JVI.02197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herbein G, Khan CA. Is HIV infection a TNF receptor signalling-driven disease? Trends Immunol. 2008;29(2):61–67. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Kosub DA, et al. Gamma/Delta T-cell functional responses differ after pathogenic human immunodeficiency virus and nonpathogenic simian immunodeficiency virus infections. J Virol. 2008;82 (3):1155–1165. doi: 10.1128/JVI.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rutjens E, et al. Differential NKp30 inducibility in chimpanzee NK cells and conserved NK cell phenotype and function in long-term HIV-1-infected animals. J Immunol. 2007;178 (3):1702–1712. doi: 10.4049/jimmunol.178.3.1702. [DOI] [PubMed] [Google Scholar]
- 73.Diop OM, et al. Plasmacytoid dendritic cell dynamics and alpha interferon production during simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82(11):5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brenchley JM, et al. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7 (3):235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 75.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12 (12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 76.Ferrick DA, et al. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373 (6511):255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 77.Martini F, et al. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vgamma9Vdelta2 T cells in chronically infected patients undergoing structured treatment interruption. J Infect Dis. 2002;186 (6):847–850. doi: 10.1086/342410. [DOI] [PubMed] [Google Scholar]
- 78.Poggi A, et al. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood. 2004;103 (6):2205–2213. doi: 10.1182/blood-2003-08-2928. [DOI] [PubMed] [Google Scholar]
- 79.Schindler M, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125 (6):1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]