Abstract
This review article compiles the characteristics of resin based dental composites and an effort is made to point out their future perspectives. Recent research studies along with few earlier articles were studied to compile the synthesis schemes of commonly used monomers, their characteristics in terms of their physical, mechanical and polymerization process with selectivity towards the input parameters of polymerization process. This review covers surface modification processes of various filler particles using silanes, wear behaviour, antimicrobial behaviour along with its testing procedures to develop the fundamental knowledge of various characteristics of resin based composites. In the end of this review, possible areas of further interests are pointed out on the basis of literature review on resin based dental materials.
Abbreviations: Bis-GMA, Bisphenol A-glycidyl methacrylate; Bis-EMA, Ethoxylatedbisphenol-A-dimethacrylate; BPA, Bisphenol-A; BPO, Benzoyl peroxide; CQ, Camphorquinone; DC, Degree of conversion; DHEPT, Dihydroxy ethyl-para-toluidine; DMAEMA, Dimethyl amino ethyl methacrylate; DMAP, Dimethyl amino pyridine; 4-EDMAB, Ethyl-4-dimethyl amino benzoate; EGDMA, Ethylene glycol dimethacrylate; HEMA, 2-Hydroxyethyl methacrylate; LED, Light emitting diode; γ-MPS, 3-(Trimethoxysilyl) Propyl Methacrylate; PPD, 1-phenyl-1,2 propanedione; PS, Polymerization Shrinkage; RBCs, Resin based composites; TEG, Triethylene glycol; TEGDMA, Triethylene glycol dimethacrylate; TPO, Diphenyl phosphine oxide; UDMA, Urethane dimethacrylate
Keywords: Dental composites, Surface modification of filler particles, Wear, Self-healing, Antimicrobial properties
1. Introduction
In recent years, demand of bio-medical materials have been increased due to increased and productive interaction of interdisciplinary fields of material science and molecular biology. Materials used in dentistry have evolved a lot with such interaction. Development of dental materials with reduced polymerization shrinkage (PS) and better depth of cure or degree of conversion [1] along with improved mechanical properties and aesthetic are of the prime interests for the researchers of dentistry and material scientists. Micro and nano-sized reinforcing fillers are considered as the most important changes that makes the dental material easy to polish and possesses higher wear resistance. Cases of tooth loss due to any reasons such as trauma, brushing habits or due to caries is a common issue which requires restoration of tooth through materials which possesses the properties as required for the dental materials. Now a days, different dental materials have been developed such glass ionomer cements (GICs) [2], silicates [3], resin based composites [4] etc. Among these dental restorative materials, resin based composite materials have been widely used restorative dental material due to their ability to withstand high compressive forces in mouth along with good aesthetic properties [5]. Monomers like Bis-GMA, TEGDMA, UDMA, HEMA, Bis-EMA have been developed and various fillers of varying particle sizes have been reinforced in these resin monomers to achieve the better aesthetic, mechanical and wear properties of the dental materials. The molecular structure of commonly used monomers in RBCs are shown in Fig. 1. These resin matrix materials get polymerized using photo-initiators such as CQ with DMAEMA or EDMAB as co-initiators when exposed to blue visible light source such as blue light emitting diodes (LEDs). Less shrinkage, high degree of conversion and depth of cure along with good mechanical, wear properties and some specific characteristics such as remineralization [6] and antimicrobial properties [7] are desired for dental restorative materials.
Fig. 1.
Molecular structure of different monomers used in RBCs.
This article critically reviews the various monomers used as dental materials, their polymerization process. Effect of different fillers and their surface modification has been discussed along with other important characteristics of dental materials such wear behaviour, antimicrobial behaviour and bio-compatibility behaviour of the different resin based dental materials. From the review of various previous studies in the field of resin based dental composite materials, some conclusions have been drawn which may led to future courses of investigations.
1.1. Prior state of art
Since 1930, methacrylate based resin has been used in denture bases [8] and were hardened through heat-curing. Later in 1940s, cold curing process was developed by German researchers which results in curing of resin in the oral cavity directly. However, high shrinkage of 21% of pure monomers of methacrylate was the major drawback of this resin [9]. Later, pre-polymerized beads were introduced in resin which successfully reduced the shrinkage to 3.5% but still shrinkage associated with these methacrylate resins were the major drawback. To deal with the excessive shrinkage issues in dental restorative material, Dr Raphael Bowen mix quartz particle with epoxy resin which showed good results, in vitro, but didn’t cure when used clinically due to sensitive setting reaction of epoxy resin for moisture contamination. Later, Dr Bowen replaced epoxy resin with methacrylate to produce dimethacrylate known as Bis-GMA or “Bowen’s Resin” which has been used as most commonly used resin material, since 1960 [10].
2. Monomers used in resin based dental materials
2.1. Bisphenol A-Glycidyl methacrylate (Bis-GMA)
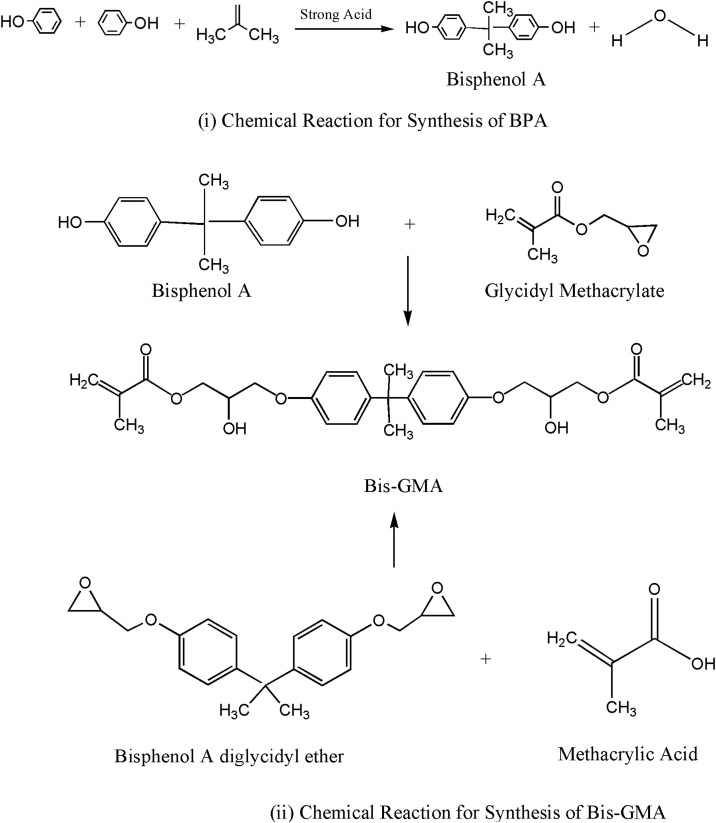
Bis-GMA consist of Bisphenol A (BPA) and glycidyl methacrylate [11]. BPA was first reported in 1891 by a Russian chemist Aleksandr P. Dianin through the chemical reaction shown in Fig. 2 (i), and was synthesized in 1905 by Zincke through condensation of acetone with two equivalents of phenol, in the presence of an acid catalyst such as hydrochloric acid (HCl) or sulphuric acid [12]. BPA [(CH3)2C(C6H4OH)2] is a colourless solid, crystalline, organic and synthetic compound belonging to group of bisphenol and diphenylmethane derivative having two hydroxyphenyl groups [13]. It is poorly soluble in water but is fairly soluble in various organic solvents. Since 1957, it is commercially used for the synthesis of epoxy resin and polycarbonates. In 1950, scientists combined BPA with phosgene (carbonyl chloride) to produce clear hard resin polycarbonate which is commonly used for production of plastic [14]. The reaction product of BPA with epichlorohydrin produced epoxy resin.
Fig. 2.
Chemical reactions for synthesis of (i) BPA, and (ii) Bis-GMA).
Bis-GMA (2, 2-bis [4-(2-hydroxy-3- methacryloyloxypropoxy)]-phenyl propane) or bisphenol A-Glycidyl methacrylate (Molecular Weight: 512) is a reaction product of methacrylic acid and diglycidyl ether of bisphenol A (BADGE or DGEBA) or alternatively through addition reaction of bisphenol A and glycidyl methacrylate as shown in Fig. 2 (ii). Bisphenol A diglycidyl ether is a colourless, solid organic compound used as constituent of epoxy resins. Its melting temperature is slightly above the room temperature. It is a reaction product of o-alkylation of BPA with epichlorohydrin. Bis-GMA has a stiff central core of phenyl ring and the two pendant hydroxyl groups, which are responsible for its extremely high viscosity (η = 1200 Pa.s) and low mobility. Hydroxyl groups increases its high water sorption capacity [15] and central core of phenyl ring has strong hydrogen bonding due π-π interaction [16]. It is most widely used monomer in the field of resin based dental materials [17]. Higher viscosity of Bis-GMA raises handling issues and is responsible for lesser degree of conversion of the monomer [18]. Advantages of using Bis-GMA over the other small-sized dental monomers, such as methyl methacrylate, include less shrinkage, higher modulus and reduced toxicity due to its lower volatility and diffusivity into tissues [19]. In order to deal with high viscosity of Bis-GMA, it must be thinned by using more flexible dimethacrylate monomers, i.e. tri-ethylene glycol dimethacrylate (TEGDMA) [20]. Due to strong hydrogen bonds between hydroxyl groups, Bis-GMA monomer shows fair impact strength even having polymer morphological heterogeneity [21]. Its value of shrinkage is reported as 5.2% [22]. Bis-GMA is a good choice to be used in dental composites as it has higher refractive index as compared to other monomers as shown in Table 1.
Table 1.
Basic properties of resin monomers.
| Monomer | Molecular Weight | Concentration of double bonds (mol/kg) | Viscosity (Pa.s) | Refractive Index | Density |
|---|---|---|---|---|---|
| Bis-GMA | 512.59 | 3.9 | 700 | 1.5497 | 1.16 |
| Bis-EMA | 540 | 3.7 | 3 | 1.532 | 1.12 |
| TEGDMA | 286.3 | 6.99 | 0.05 | 1.46 | 1.09 |
| UDMA | 470 | 4.25 | 8.5 | 1.485 | 1.12 |
| HEMA | 130.14 | * | * | 1.452 | 1.07 |
| PPGDMA | 600 | * | 0.09 | 1.45 | 1.01 |
Not Known.
2.2. Ethoxylated bisphenol a glycol dimethacrylate (Bis-EMA)
By replacing pendant hydroxyl group of Bis-GMA with an epoxy specie (CH2−CH2-O-), it becomes ethoxylated Bis-GMA knowm as Bis-EMA, a hydrophobic analog of Bis-GMA used in many restorative resin dental material. For the ethoxylation reaction, ethylene oxide which is very reactive molecule, is generally used. The major advantages of Bis-EMA are its low viscosity, low water sorption and low polymerization shrinkage which attributes it to be used as diluent in resin matrix in place of TEGDMA. Like Bis-GMA or TEGDMA, it is not a monomer but a large homologous dimethacrylate molecules of ethoxylated BPA [23]. Due to steric hindrances, its degree of conversion of double bond is low despite its low viscous nature [19].
2.3. Triethylene glycol dimethacrylate (TEGDMA)
Oxidation of ethylene in presence of silver oxide (AgO2) catalyst at high temperature forms ethylene oxide which after hydration gives TEG as reaction product. Methacrylic acid when reacts with triethylene glycol (TEG) forms a dimethacrylate known as TEGDMA. In this reaction, the methacrylate groups bond to each end of the TEG which forms TEGDMA. It is frequently used as a diluent in dental composites due to its low viscosity. The polar bond interaction between chains of TEGDMA is weak and backbone structure is quite flexible which leads to low viscosity. TEGDMA is a long molecule having two functional methacrylate groups at terminals similar to Bis-GMA. However, between the two methacrylate groups, there is a linear chain hence it has lesser viscosity as compared to Bis-GMA. Due to lesser viscosity, it is used as a diluent for the Bis-GMA, improving the handling of the composite resin along with incorporating higher filler loading [20]. However, the major disadvantages of adding TEGDMA to resin matrix are (i) High water sorption, (ii) Reduced mechanical properties, and (iii) low colour stability.
During polymerization, Bis-GMA and TEGDMA forms a highly stable acrylic bonds with inorganic filler particles through coupling agent grafted on these filler particles. A tridimensional network which possess good mechanical and chemical characteristics, is thus formed.
2.4. Hydroxyethyl methacrylate (HEMA)
The synthesis of 2-hydroxyethyl methacrylate (HEMA) and its polymerization process was described in U.S. Patent 2,028,012, 1936 [24] and applications as hydrogels was reported in 1960 [25] while it studied fundamentally in 1965 [26]. It can be synthesized from methacrylic acid through transesterification reaction of ethylene glycol (Scheme 1) or through reaction of ethylene oxide and methacrylic acid (Scheme 2) [25] as shown in Fig. 3. Products prepared by these two methods contains impurities in various percentages: e.g., methacrylic acid formation due to hydrolysis reaction of HEMA and EGDMA resulting from esterification reaction between methacrylic acid or HEMA and ethylene glycol. It is a low molecular weight monomer, which is characterized by its hydrophilic properties and is an important constituent of most adhesive systems.
Fig. 3.
Chemicals reactions for synthesis of HEMA through different schemes.
2.5. Urethane dimethacrylate (UDMA)
UDMA was developed by Foster and Walter in 1974 [27]. It was synthesized from hydroxyalkyl methacrylates and disocyanates. 1, 6-bis (methacrylyloxy–2-ethoxy–carbonyl amino) -2, 4, 4 trimethythexane. It is a reaction product of 2-hydroxyethyl methacrylate and 2, 4, 4- trimethyl- hexamethylenediisocyanate as shown in Fig. 4. The absence of a phenol ring in the monomer chain leads to higher flexibility and toughness in comparison to Bis-GMA. Because of the significantly lower viscosity (100 times lesser than Bis-GMA) and therefore higher mobility of UDMA, Bis-GMA was replaced, either partially or totally, by this rather new monomer in many commercially available dental composite resin materials [27].
Fig. 4.
Chemicals reaction for synthesis of UDMA.
UDMA shows a higher viscosity than TEGDMA and Bis-EMA, due to the hydrogen bonding between the –NH– and carbonyl groups, which, however, is much lower than the viscosity of Bis-GMA, because imino groups form weaker hydrogen bonds compared to hydroxyl groups [28]. UDMA contains an aliphatic spacer group between the methacrylates, but exhibits a relatively high glass transition temperature (Tg), due to the presence of the urethane groups (–NHCOO–) which contain rigid quasi-conjugated double bonds and also forms hydrogen bonds [19]. The UDMA-based resins have greater polymerization rate and degree of conversion as compared to Bis-GMA based resins [29]. Despite its low viscosity as compared to Bis-GMA, UDMA shows lower mobility, low reactivity and low degree of conversion when it is mixed with Bis-EMA [30].
3. Polymerization of resin matrix
The linear molecules having a methacrylate group at ends are most widely used monomers in resin based dental composites. Chain growth polymerization is responsible for the conversion of monomers to polymers through polymerization in three phases i.e. initiation, propagation and termination. Free radicals are formed by photo initiators in case of most of the methacrylate based composites.
Polymerisation process is initiated by the generated free radicals during photoinitiation which converts C C bonds into C—C bonds between generated radical and methacrylate group of the monomer molecule. Radical and alkene group of methacrylate donates an electron. Remaining electron of the alkene group reaches the opposite terminal of the monomer and hence the whole molecule becomes a radical and reacts with another monomer. This results in chain reaction which ends up when two radicals reacts with each other. Maximum conversion of uncured resin into cured/polymerized resin increment is required which is measured as Degree of Conversion (DC) of resin.
Apart from the above simpler situation of polymerization, dental composites have multiple dimethacrylate monomers resulting in highly cross linked polymers with better mechanical and wear resistance property. Photo-initiators have either low energy bond which gets cleave upon absorption of light or have excitable chemical group which reaches an excited electron state due to absorption of light. TPO and BPO are the former type I photoinitiators which have low energy bonds which upon homolytic cleavage yields two radicals which initiate polymerization. These photoinitiators have strong absorption near ultra violet (UV-A) and some overlap with visible light [31]. They doesn’t require co-initiators and are less yellow and more off white but turns yellow after polymerization when used at high concentrations [32]. Quartz Tungsten-Halogen lamps are used as light curing unit which are not so compact. In latter type II photo-initiators, co-initiators are required to yield radicals. Co-initiators are generally tertiary amines which have nitrogen atom with three chains and donates the proton to the highly excited initiator to form free radical. Upon exposure to blue visible light, electron exchange in initiator-coinitiator, yields radicals through hydrogen abstraction [31]. Initiator molecule becomes a ketyl radical while co-initiator molecule becomes an amino alkyl radical able to initiate the polymerization reaction. This process of photo polymerization is illustrated in Fig. 5.
Fig. 5.
Photo-initiation by hydrogen abstraction (Type II).
3.1. Common initiators used for photo polymerization
Dental resins were cured by principle of photo polymerization in around 1975. Photo polymerization process starts due to photo-initiation system which comprises photo-initiator and an electron donor or tertiary amine. Molecular structure of commonly used photo-initiators are shown in Fig. 6. A yellow powder camphorquinone (CQ) is most commonly used photo initiator along with electron donor tertiary amine DMAEMA and EDMAB as co initiator. CQ is preferred to be used as photo-initiator due to its broad absorbance range of 360–510 nm and peak absorbance at 468 nm in visible light spectra. Bathochromic shift (Lengthening of peak absorbance wavelength) to 474 nm occurs when CQ is dissolved in monomers like TEGDMA. Blue light emitting diodes (LED) are most commonly used LCUs having wavelength range of 400–500 nm. Major disadvantages of using CQ is its yellow colour which may be bleached out by LEDs during polymerization [33]. A study shows that it can alter metabolism of structural lipids which affects membrane integrity and permeability [34]. Such concern may raise the thoughts of working on replacements for CQ in future researches.
Fig. 6.
Molecular structures of different photo-initiators (i) CQ, (ii) TPO, and (iii) PPD.
A pale yellow liquid 1-phenyl-1, 2 propanedione (PPD) is a α-diketone which forms free radicals using both type I and type II reactions. Light absorbance range is 300–400 nm with peak at 410 nm. Unlike two carbonyl radicals of CQ, free radicals of PPD did not recombine resulting its prolonged action as compared to CQ. Significant improvement in depth of cure has been reported when CQ and PPD were combined in the ratio of 1:1 and 1:4 [35]. Improvement in mechanical properties and degree of conversion have been reported using PPD as compared to CQ [31]. As the absorbance range of PPD is similar as that of conventional LEDs, it can be used along with CQ for photo-polymerization process.
3.2. Co-initiators
Amines are used as co-initiators or accelerators to accelerate the polymerization through proton and electron transfer via initiating radicals. One of the most commonly used co-initiator is N, N-dimethyl-p-toluidine (DMPT) but is reported to be toxic due to its relatively lower molecular mass [36]. Ethyl-4-(dimethylamino) benzoate (4-EDMAB) has also been used as co-initiator as it donates hydrogen but is reported cytotoxic due to being aromatic amine and it cannot be polymerize with monomers [37]. 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA) has methacrylate group which helps it to polymerize along with the monomers and hence has an advantage that it doesn't leach out and has better biocompatibility.
N, N-dimethyl-p-toluidine (DMPT), ethyl-4-dimethylaminobenzoate (4-EDMAB), 2-ethyl-dimethyl benzoate and N-phenylglycine, p-octyloxy-phenyl-phenyl iodonium hexaflu- oroantimonate (OPPI) can be used in combination with CQ as an accelerator [60]. Molecular structure of commonly used co-initiators are shown in Fig. 7. Basic properties of commonly used initiators and co-initiators are shown in Table 2.
Fig. 7.
Molecular structures of different co-initiators (i) Tertiary Amine (ii) DMPT, DMAEMA, and (iv) EDAB.
Table 2.
Basic Properties of Initiators and Co-initiators.
| Type | Name | Molecular weight | Refractive index | Density (g/cm3) | Absorbance (nm) |
|
|---|---|---|---|---|---|---|
| Range | Peak | |||||
| Initiator | CQ | 166 | * | 0.97 | 360-510 | 474 |
| Initiator | TPO | 348 | 1.48 | 1.12 | 230-430 | 385 |
| Initiator | PPD | 148 | 1.53 | 1.1 | 300-480 | 410 |
| Co-initiator | DMAEMA | 157 | 1.44 | 0.93 | NA | |
| Co-initiator | DMPT | 135 | 1.54 | 0.94 | NA | |
| Co-initiator | EDAB | 193 | 1.53 | 1.06 | NA | |
| Co-initiator | Na-NTG-GMA | 329 | * | * | NA | |
NA: Not Applicable.
Not Known.
3.3. Factors affecting polymerization of RBCs
Through polymerization, achievement of maximum degree of conversion of resin materials has been a challenge for the researchers. There are various factors which affects the polymerization process of resin based composites such as composition of RBCs, curing mode, light curing time, increment thickness, light curing units used, post-irradiation time, cavity diameter and its location, distance of light curing tip from surface, substrate used, type of filler, and temperature. Increased filler-matrix ratio leads to progressive fall of degree of conversion as filler particles acts as obstacle in chain propagation of polymers [38]. Filler's light permeability, monomer composition, initiator concentration and co-initiator/inhibitor system of RBCs also affects its depth of cure and hence, degree of conversion significantly [39]. Extended curing time leads to better polymerization properties of bulk-fills in deep cavities [40]. Many studies (Table 3) have been carried out to study various parameters and their effect on polymerization properties. It is evident from these studies that there are various parameters which affects polymerization properties and hence affects other related properties.
Table 3.
Factors affecting Polymerization of Resin Based Composites.
| Author | Year | Resin material | Factor affecting polymerization | Properties affected by Polymerization | Results |
|---|---|---|---|---|---|
| Dimitrios Dionysopoulos et al. [41] | 2016 | X-tra fil–XF, EverX Posterior–EXP, Tetric EvoCeram Bulk Fill–TEB and Beautifil Bulk Restorative–BBR, X-tra base–XB, Beautifil Bulk Flowable–BBF, Filtek Bulk Fil–FB and Venus Bulk Fill–VB, Filtek Z550 – FZ | Composition, Temperature and Post-Irradiation Curing | Microhardness | Depth of cure < 4 mm. Preheating at 54 °C increases microhardness. After 24 h, microhardness increses due to post-irradiation polymerization. |
| Tae-Sung JEONG et al. [42] | 2009 | Z250, Solitaire 2 | Resin shades | Reflectance (%R) and absorbance measurements, Microhardness, PS, Color change. | Resin shades had minimal effect on microhardness, polymerization shrinkage, and color change. Efficient of the incident photons were not consistently correlated. |
| Pfeifer et al. [43] | 2008 | Formulation B (Equal parts of Bis-GMA, TEGDMA), Formulation U (Equal parts of Bis-GMA, TEGDMA, UDMA) | Three irradiances- 220, 400, or 600 mW/cm2 | Volumetric shrinkage, DC, Polymerization rate (RPmax) | Polymerization reaction rate and shrinkage were not correlated. Irradiance affected polymerization reaction rate and stress development. Lowest RPmax corresponds to highest stress/degree of conversion. |
| Harahap et al. [44] | 2017 | Filtek Z 350XT | Bench time | Depth of Cure | Bench time of 60 min after removal from the refrigerator has the greatest depth of cure. |
| Gabrielle Ribeiro Lima Muniz et al. [45] | 2013 | FillMagic enamel A3, 20 with the hybrid resin Filtek P60 A3 and 10 with the indirect resin Epricord Enamel E1 | Heat Treatment | Water sorption and solubility tests | Heat treatment reduced the sorption and solubility values. |
| Sayna Shamszadeh et al. [46] | 2016 | Tetric EvoCeram universal, Tetric EvoCeram bulk-fill | Increment Thickness | Color Stability | Color change > conventional after coffee staining and is also a function of increment thicknesses. |
| Flávio Henrique Baggio Aguiar et al. [47] | 2005 | Z250 | Curing tip distance (2 mm, 4 mm, and 8 mm), RBC shade (A1, A3.5, and C2) | Knoop microhardness | Top surface showed higher hardness than the bottom surface, A1 showed highest microhardness followed by A3.5 and C2. |
| Pnina Segal et al. [48] | 2015 | Filtek Ultimate Universal Restoration, shade A2 dentin, Empress Direct, shade A2 dentin | light intensity of LED, QTH curing devices in relation to the light distances | Hardness | Increasing the distance of the light source, the light intensity and the microhardness values at the top and bottom surface decreases. Greater Microhardness at the bottom surface. Filtek Ultimate (3 m) showed highest microhardness values. |
| Palin et al. [49] | 2014 | 50/50mass% of Bis-GMA and TEGDMA | curing protocol was varied (400mWcm−2 for 45 s, 1500mWcm−2 for 12 s and 3000mWcm−2 for 6 s | DC, Polymerization rate, Flexural strength, Polymerization stress, Cuspal deflection, microleakage | Phosphine oxide initiator provided superior mechanical and physical properties for high irradiance curing protocols compared with materials based on camphoroquinone |
| Seyed Mostafa Mousavinasab et al. [50] | 2014 | Filtek Z250, Filtek P90 | distance from light curing source (0 mm or 2 mm) | Hardness | Hardness decreased as the distance increased and Filtek Z250 showed higher hardness compared to Filtek P90. |
4. Fillers used in dental materials
4.1. Classification of fillers in dental composites
Resin based dental composites are broadly classified on the basis of handling and filler composition. Flowable composites contains monomers such as HEMA which reduces viscosity without sacrificing filler loading while packable composites are quite viscous and contains diverse filler sizes which makes them strong.
Filler materials can also be classified on the basis of particle size as well. First generation of filler materials were “Macrofill” with particle size of 10–50 μm but their high wear rate and low polishing leads to poor aesthetics. Second generation “Microfill” composites with fumed silica particles of ≈40 nm were developed which have better wear and polishing properties but lacked in strength due to low filler loading. Later, “Hybrid” composites were developed with particle sizes of “Macro” and “Mini” to get the optimal set of strength and aesthetic properties. With advancements in milling techniques, “Minifill” composites were developed with particle size of 0.4–1 μm along with fumed silica particles. “Microhybrids” were developed to get the good wear, polishing and aesthetic properties. From the last decade, “Nanofill” composites become an important advancement in the field of resin based dental composite material. Particle size of 5–100 nm not only provides better polishing and aesthetic properties to dental composites but also better wear properties because they didn't form large pores when a particle wear out from it. Nanoparticles makes very strong bonds with themselves and other particles due to their high free surface energy. Agglomeration of nanoparticles can be reduced by functionalizing nanoparticles which produces similar surface charge and hence nanoparticles repels each other. It is reported in many studies that incorporating nanoparticles in resin based dental material improved its flexural, fracture toughness and adhesion to tissue. Most of the studies showed that incorporating inorganic nano-filler in resins improved some of the mechanical properties as compared to unfilled resins.
4.2. Effects of surface modified fillers on properties of dental composite resin
Inorganic nanoparticles may agglomerate and hence may distribute non-uniformly in dental composite. Hence to achieve homogeneous distribution of filler particle, various surface modifications techniques of filler particles have been suggested to get optimal compatibility of filler particles with resin phase. Physical surface modification can be achieved through ultraviolet laser and fume oxidation but these techniques are not so popular in dental materials. Chemical modifications alters surface of filler particles through chemical reaction between inorganic filler particles and small molecules such as silane coupling agent or grafting polymeric chain or brushes through covalent bonding to the hydroxyl groups existing on the particles. Durability of dental composite can significantly be improved by improving inter-facial properties and hence quality of interface plays a pivotal role in improving degree of conversion, dispersion of filler particles, filler loading amount and mechanical properties of dental composite [51]. Among coupling agents like zirconate, titanate and silanes, zirconate are quite reactive and hence are used for those surface which doesn't contain reactive hyroxyl group. Fine adhesion between resin and zirconia filler particles have been reported in studies using zirconate as coupling agent [52,53]. Titanate coupling agent provides interface between resin and inorganic filler through proton coordination on non-silane substrate and doesn't require water for condensation and also forms less voids in the composite. TiO2 nanoparticles disperses uniformly in the composite using titanate as coupling agent via ultrasonic wet method [54]. Silane coupling agents are mainly organic silicides (X3SiY}) where X may be chloro, alkoxy or acetoxy group and Y may a vinyl, epoxy, amino or mercapto group. X groups converts to alkoxy group via hydrolysis and makes hydrogen bonding or covalent bonding with alkoxy group present on surface of inorganic filler particles while Y are reactive group which binds with organic resin and hence improves adhesion of interface [55]. Commonly used silane is γ-Methacryloyloxypropyl trimethoxysilane (3-MPS or γ-MPTS) which is used for the surface modifications of fillers particle in resin composite to improve inter-facial adhesion [56]. The methoxy group of γ-MPTS combines with hydroxyl group on the filler surface and other silane molecules and copolymerizes via methacryloxy functional groups with methacrylic resin polymer matrix [57]. It is hydrophilic and has lesser hydrolyzability hence all alkoxy groups doesn't react with hydroxysilyl group which results uneven surface modifications of filler particles.
Silane treatment improves hydrolytic stability by preventing water to enter the silica-matrix interface [58]. Many studies utilized hydrophobic functional groups such as vinyl and phenyl and reported improvement in hydrolytic stability of composite. Craig et al. [59] utilized mixtures of fluoroalkyl-, aminoalkyl‐, phenyl‐, vinyl‐, bis silyl ethane‐ and 3-methacryloxypropyltrimethoxysilane (MAOP) to improve hydrophobicity of coupling agent and reported that vinyltriethoxysilane improved the hydrolytic stability of the composite significantly. Fig. 8 demonstrate the surface modification process of inorganic filler particle using silane treatment.
Fig. 8.
Surface modification of inorganic filler (Silane treatment).
5. Characteristics of dental materials
5.1. Wear behaviour of resin based dental materials
Undesired removal of constituent particles from a material due to any mechanical interaction is known as Wear. There are mainly three types of wear processes in dental materials, i.e., Attrition, Abrasion and Erosion. Loss of dental material due to physical contact between occlusal surfaces of tooth is known as attrition while loss of dental material due to interaction of tooth with other material is known as abrasion. Dissolution or acid-based leaching of hard tissues is known as erosion. Patients having bruxing and clinching habits are most prone to the wear of tooth material or restorations [60].
Oral environment is quite complex and contains different parameters which can lead to wear of teeth or restorations such as different chewing load, food slurry, salivary variables and tooth brushing habits. Heintze et al. [61] studied the surface deterioration of RBCs due to tooth brushing time and load using simulation device replicating tooth brushing phenomenon was employed with tooth paste slurry and three different load conditions. Through ANOVA, it was revealed that material, load and brushing time significantly affect wear of teeth material. Many researches have carried out studies to measure the varying chewing loads through different approaches. Another important factor influencing oral environment is saliva (pH7) which can modulate erosive/abrasive tooth wear through formation of pellicle and by remineralization but cannot prevent it [62]. Acidic diets (usually of pH3), soft drinks (pH1 to pH6) and regurgitated gastric acid (pH1.2) may cause significant wear of teeth or restorations. Sonal et al. [63] studied effect of silane treated nano silica (0–9 wt%) on wear characteristics of RBCs using two body wear abrasive test in artificial saliva medium. Minimum wear of dental composites were found at 9 wt% of nano silica as filler. Composites which were thermo-cycled and immersed in tea were reported for maximum elastic modulus and hardness. Gan et al. [64] evaluated five Bis-GMA resin based dental composites for friction with wear performance in different oral environment medium such as distilled water, artificial saliva and soft drink. Using a ball on flat rig, two body type wear test was performed and better wear resistance was foun for filtek P60 as compared to other nano-filled dental composites. For VITA ZETA (under distilled water) dental composites, minimum coefficient of friction at steady state condition was found. Hu et al. [65] also investigated wear characteristic of dental materials using sliding wear test on pin on disk apparatus using artificial saliva as medium of oral environment. Steatite ceramic was employed for abrasive counter face. P-60 visible light cured RBCs and Au-Pd alloy dental material showed maximum and minimum volume wear loss, respectively. However, Au-Pd alloy dental material and P-60 light cured composite showed maximum and minimum volume wear loss on abrasive ceramic wheel, respectively. Chadda et al. [66] evaluated fracture toughness and wear performance of Bis-GMA/TEGDMA (69.5/29.5 wt%) reinforced with hydroxyapatite (micro-filled) and silica/hydroxyapatite (micro-hybrid filled) for dental restoring composites. Wear performance test (sliding) was performed using pin-on-disk tribometer. In descending order, sliding wear rate was observed as H50>H0>H20>H40 > H30 and SH50 > SH0 > SH20 > SH30 > SH40.
Altaie et al. [67] compared tribological behavior of six different resin based composites (RBC) using pin on disk wear test rig and modified pin on disk rig. Rectangular bar specimens after wear test were scanned by profilometry to study the wear tracks. The found the maximum mean volume loss (mm3) in filtek supreme RBC.
Souza et al. [68] investigated abrasive and sliding wear behaviour of four commercial dental restorative composites using reciprocating sliding test. Resins employed were Bis-GMA, Bis-EMA, UDMA and TEGDMA along with barium glass and colloidal silica as inorganic filler. in ratio of 18:82. The minimum wear volume through reciprocating sliding was found to be 0.3 mm3 in composite C (Resin to Filler ratio of 18:82) which also showed minimum wear volume through micro abrasion test. Zhi et al. [69] compared four CAD/CAM resin based composites and dental ceramics. Mastication simulator was employed to test wear rate at 2,00,000 cycles and thermal cycles of 500 against natural human enamel. The results showed that maximum and minimum mean wear of composite resin was found in Kerr experiment material (87.20 ± 35.036 μm) and 3 M Lava ultimate (61.90 ± 35.070 μm), respectively. Mean wear value in ceramic (Vita mark II) was reported as 12.10 ± 8 μm.
Through different in-vitro and in-vivo studies, many attempts have been made to understand the complex wear mechanism to access the wear of teeth and restorations. For the in-vitro studies, many machines have been developed by simulating the oral environment including loading cycles during mastication such as simple pin-on-disc sliding machine [70], reciprocating wear test rigs [60] to measure the two and three body wear mechanism of tooth material and restorations. There is a challenging area for further studies to be carried out to study the wear of different resin based dental materials in varying oral environment.
5.2. Self-healing resin based dental materials
Half of the resin based dental materials before 10 years from their restoration and 25% of the resin based dental materials fails due to fracture [[71], [72], [73]]. Mastication forces and thermal stresses forms micro-cracks and leads to failure of dental composites [74]. To repair the developed cracks, self-healing characteristics of various polymers have been utilized in recent years. These characteristics have shown the new directions in the field of bonding durability of dental materials. These self-healing materials are capable of repairing the crack and hence dental materials regains its load bearing capabilities. Generally, microencapsulation of these self-healing liquid in composites has been used to provide self-healing characteristics to them. In case of crack or damage at the site of restoration, microcapsules after rupture, releases the healing liquid which flows into the cracks and gets polymerized due to catalyst to repair the crack by filling the crack together. White et al. [75] in 2001 reported the pioneer successful mechanism of self-healing in polymers by encapsulation of dicyclopentadiene (DCPD) in a shell of poly(urea-formaldehyde) (PUF). For polymerization of released DCPD, Ruthenium-based Grubbs' catalyst in epoxy matrix was used. They yield 75% fracture toughness with the help of self-healing mechanism in their study. Since then, many researchers have reported the self-healing characteristics in various polymers using different compositions of self-healing agents and different shells for encapsulation. Brittany E. Wertzberger et al. [76] replicated White’s approach to investigate the efficacy of self-healing of DCPD and Grubbs' catalyst in resin based dental composites in terms of its fracture toughness. The results of their study yield recovery of 57% fracture toughness using self-healing approach. Sonja Then et al. [77] encapsulated DCPD in melamine-modified UF microcapsule and neat UF microcapsules shells to access the mechanical properties of resin based dental composite with self-healing microencapsulations. They reported that mechanical properties of resin based dental composites containing self-healing microcapsules were not affected. Biocompatibility concerns of DCPD and Grubbs catalyst and their high cost, no further use of these materials were reported to develop self-healing resin based dental composites [78,79]. In a another study, TEGDMA liquid in polyurethane (PU) shell was used as self-healing agent without any catalyst but it doesn’t showed self-healing characteristics [80]. Later, Junling Wu et al. [81] synthesized microcapsules of polymerizable monomer based self-healing liquid TEGDMA and DHEPT in PUF shell. They reported 65% efficiency of this self-healing microcapsules. Qi Li et al. [82] evaluated microcapsules of liquid curing agent, polyetheramine as core material and PMMA as shell material to study the effect of different process parameters on self-healing and mechanical properties of epoxy based composites.
In an another study [83], SHDC containing microcapsules of TEGDMA and DHEPT in PUF shell (0%, 2.5%, 5%, 7.5% and 10% wt%) were evaluated for mechanical properties and self-healing efficiency with 1 day to 6 months of water-aging of SHDC. They concluded that SHDC containing 7.5% of microcapsules showed effective self-healing efficacy without affecting mechanical properties. Chen Chen et al. [84] developed and evaluated 2-methacryloyloxyethyl phosphorylcholine (MPC) dental composites with 10% microencapsulation of TEGDMA and DHEPT in PUF for protein adsorption. MPC is a water soluble, biocompatible and strong protein repellent polymer which reduces the adhesion of oral pathogenic microorganisms [85]. Recently, Mobin Yahyazadehfar et al. [86] evaluated fracture resistance and healing capacity of SHDC under monotonic and cycling loading of methacrylate silane (MA-silane) and H-bonding forming hydroxyl silane (OH-silane) treated microcapsules in different weight percentage. They concluded that MA-silane treated SHDC showed best healing efficiency and fracture toughness at 5 wt% microcapsules. Polyurethane microcapsules were also questioned as these were fractured when mixed with silica particles due to larger difference in physical strength. George Huyang et al. [87] prepared microcapsules through silicate condensation to develop SHDC with 25% healing efficiency and promising mechanical properties. In another study, George Huyang et al. [88] developed potential SHDC through combining clinically acceptable glass ionomer cements and self-healing model given by White et al. [75], together.
Investigation of mechanical strength and stress concentration in interface between capsule and matrix has been an area of interest for researchers. F.A. Gilabert et al. [89] predicted the stress concentration and mechanical strength of interface using the model based on finite element analysis and cohesive surface technique of Abaqus. Developing dental materials which possesses triple properties i.e. self-healing, antimicrobial and remineralization is also a new area of interest. Shichao Yue et al. [90] developed novel dental composites which possesses self-healing, antimicrobial and remineralization capabilities. Healing precursors may reach submicrons level through nano encapsulation. So, development of novel encapsulation technique may give new dimensions to SHDCs.
In the field of dental adhesion, self-healing bonding resins may provide a new direction for the improvement of the bonding durability. To allow the healing precursors in dental adhesives to reach the submicron spaces created by acid etching within dentin, the need for nanoencapsulation is needed.
5.3. Antimicrobial behaviour of resin based dental materials
Most commonly, secondary dental caries are responsible for the failure of restorations [91]. They are caused predominately due to the activities of microorganisms on the gingival surfaces. So, developing a new class of materials which can eliminate or reduce bacterial acids is one of the prime interest of many researchers in the field of dentistry and biomaterial science.
5.3.1. Methods for testing antimicrobial properties of dental materials
Many methods have been used in various researches for the assessing antimicrobial properties of dental material. Agar disc diffusion test is the most commonly used method due to its low cost and simplicity. In this test, samples in the form of tablet are placed on the inoculated agar plate surface. Antimicrobial activity of leached out soluble agent is evaluated through measuring diameter of the inhibition zone on the surface of disc. This method is appropriate for the soluble agents only which can diffuse in the agar gel. As, leaching out of adequate antimicrobial agent is rare in cured composites and thus no inhibition has been found in most of the cases. So, cured restorative materials with non-leachable antimicrobial components such as quaternary ammonium methacrylates (QAM) immobilized in restorative materials can be accessed for antimicrobial test through agar-plate method or on-disc culture assay [[92], [93], [94]]. In this method, a fixed concentration of bacterial suspension is spread on bacteria cultured brain–heart infusion (BHI) broth. Then, test material to be investigated is placed on the BHI agar plate and the plate is incubated aerobically for 24 h at 37 °C. After incubation, colony-forming unit (CFU) is further determined which would evaluate the antimicrobial nature of the test material.
Imazato et al. [92] employed minimum inhibitory concentrations (MIC) method to reveal antimicrobial activity of non-polymerized monomers such as 12-methacryloyloxydodecylpyridinium bromide (MDPB). MIC is measured as the lowest amount of concentration of antimicrobial agent which can inhibit bacterial growth completely. It can be determined through visual inspection or through spectrometry. Minimum Bactericidal Concentrations (MBC) is the minimum amount of antimicrobial agents that can kill 99.9% of bacteria which doesn’t produce colonies on the surface of plate.
A newer method known as direct contact test utilizes confocal laser scanning microscopy (CSLM), fluorescence microscopy, and/or scanning electron microscopy (SEM) to interpret images of growth of microbial biofilms on disk containing resin.
5.3.2. Antimicrobial materials
One such class of materials contains antimicrobial agents which may be releasing or non-releasing type agents. Chlorhexidine (CHX) [95] and silver nanoparticles (NAg) [96] have been successfully employed as antimicrobial releasing type agents. Releasing type antimicrobial agents have short life and causes deterioration in restorations however non-releasing antimicrobial agents have longer life due to immobilization of antimicrobial non releasing agents in resins and maintains the good mechanical properties of restorations [97]. In 1994, Imazato et al. [98] first incorporated quaternary ammonium methacrylates (QAMs) as antibacterial agent in dental materials. 12-methacryloyloxydodecylpyridinium bromide (MDPB) [[99], [100], [101]], poly-quaternary ammonium salts (PQAS) have been used as antibacterial agents in restorative materials. Quaternary ammonium salts shows antimicrobial mechanism causing cytoplasmic leakage when positively charged quaternary amine (N+) comes in contact with negatively charged bacterial membrane as their interaction causes electric unbalance [102]. Molecular structures of quaternary ammonium monomers are shown in Fig. 9. Lei Cheng et al. [103] combined nanoparticles of silver, QAM and Nanoparticles of amorphous calcium phosphate (NACP) to develop dental materials with double properties of antibacterial and remineralization capabilities. Resins with NACP releases calcium and phosphate ions which causes remineralization. Yapin Wang et al. [104] synthesized novel monomer of dimethacrylate containing Bis-GMA like backbone and chelating group with zirconium − fluoride complex which behaved as antibacterial fluoride releasing monomer. In most of the studies, different antimicrobial agents have been employed but their antimicrobial activities were assessed for shorter periods. Recently, Yasaman Delaviz et al. [105] investigated antimicrobial behaviour of oligomers which were synthesized from ciprofloxacin (CF) and metronidazole (MN) against Streptococcus mutans through using human gingival fibroblasts. Results of viscosity of drug oligomers were lesser than Bis-GMA and their fracture toughness was found to be comparable with commercial dental composites. Hongyan Chen et al. [106] investigated effect of filler composition of ZnO@m-SiO2 and silanized SiO2 and their physical and mechanical properties along with their antimicrobial activity. They concluded that impregnation of ZnO@m-SiO2 and silanized SiO2 showed excellent mechanical properties with antimicrobial activity. Letícia Cristina Cidreira Boaro et al. [107] developed dental composite with Montmorillonite (MMT) and CHX, which showed significant antibacterial activity against S. mutans, P. gingivalis and S. Aureus without compromising mechanical properties. In a few studies [108,109], to achieve long term effect of antibacterial properties, immobilized bactericide filler were successfully incorporated. But still, in most of the studies, different antimicrobial agents have been employed but their antimicrobial activities were assessed for shorter periods. Materials and processes which may show antimicrobial properties for longer period is an area of key interest.
Fig. 9.
Molecular Structures of Quaternary Ammonium Monomers.
6. Future perspectives & conclusion
It is quite challenging to draw a clear conclusion about the effect of various resin monomers, filler particles on the performance characteristics of dental composites from these studies because most of the studies were carried out to study few physical properties by varying one parameter. Assessment of more number of performance characteristics with varying input parameters for the development of resin based dental materials can be studied with implementation of design of experiment (DOE) [38,110] techniques. Various factors affecting polymerization are of key importance in RBCs. Further investigation may be carried out to assess the effect of different input parameters of polymerization process to achieve the desired physical, thermo-mechanical and wear properties of RBCs. Through this literature survey, it can be said that incorporating nanoparticles yields better properties but there are concerns about the leaching of nanoparticles over a time so intensive research on effects of nanoparticles on health is to be assessed in depth. Study of wear of RBCs in complex oral environment is also a challenging area of research. Functionalized dental materials have been evolved in past ten years but longevity of specific function is needed to be explored in detail. So, it can be concluded that there is vast space for further research in terms of appropriate resin material, filler particles and their surface modifications, wear behaviour in varying oral environment and specific functionality of dental materials such as antimicrobial properties, self-heling properties, remineralization properties etc.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
Authors would like to express their gratitude to Dr. Abhijeet Singh (Associate Professor & Head, Department of Biosciences, Manipal University Jaipur, Jaipur, Rajasthan, India), Mr. Bjorn John Stephen and Ms. Surabhi Suchanti (Department of Biosciences, Manipal University Jaipur, Jaipur, Rajasthan, India) for their valuable help during compilation of antimicrobial testing procedures for this review article.
References
- 1.Jang J.-H., Park S.-H., Hwang I.-N. Polymerization shrinkage and dept of cure of bulk-fill resin composites and highly filled flowable resin. Oper Dent. 2015;40(2):172–180. doi: 10.2341/13-307-L. pMID: 25136904. [DOI] [PubMed] [Google Scholar]
- 2.Lohbauer U. Dental glass ionomer cements as permanent filling materials? Properties, limitations and future trends. Materials. 2010;3(1):76–96. doi: 10.3390/ma3010076. [DOI] [Google Scholar]
- 3.L ¨uhrs A.-K., Geurtsen W. Biosilica in evolution, morphogenesis, and nanobiotechnology. Springer; 2009. The application of silicon and silicates in dentistry: a review; pp. 359–380. [DOI] [PubMed] [Google Scholar]
- 4.Jandt K.D., Sigusch B.W. Future perspectives of resin-based dental materials. Dent Mater. 2009;25(8):1001–1006. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Chan K.H., Mai Y., Kim H., Tong K.C., Ng D., Hsiao J.C. Review: Resin composite filling. Materials. 2010;3(2):1228–1243. doi: 10.3390/ma3021228. [DOI] [Google Scholar]
- 6.Marovic D., Tarle Z., Hiller K.A., ¨uller R.M., Rosentritt M., Skrtic D., et al. Reinforcement of experimental composite materials based on amorphous calcium phosphate with inert fillers. Dent Mater. 2014;30(9):1052–1060. doi: 10.1016/j.dental.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K., Zhang N., Weir M.D., Reynolds M.A., Bai Y., Xu H.H. Bioactive dental composites and bonding agents having remineralizing and antibacterial characteristics. Dent Clin North Am. 2017;61(4):669–687. doi: 10.1016/j.cden.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink, Acrylic Dental Fillers, Reactive Polymers Fundamentals and Applications (2013) 453–474. doi:10.1016/B978-1-4557-3149-7.00019-X.
- 9.Franco A.P.G., Karam L.Z., Galv ~ao J.R., Kalinowski H.J. Evaluation of shrinkage polymerization and temperature of different acrylic resins used to splinting transfer copings in indirect impression technique. 24th International Conference on Optical Fibre Sensors, Vol. 9634. 2015 p. 96347R. [Google Scholar]
- 10.Barszczewska-Rybarek I., Jurczyk S. Comparative study of structure-property relationships in polymer networks based on Bis-GMA, TEGDMA and various urethane-dimethacrylates. Materials. 2015;8:1230–1248. doi: 10.3390/ma8031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo S., Zhu W., Liu F., He J. Preparation of a Bis-GMA-Free dental resin system with synthesized fluorinated dimethacrylate monomers. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17122014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalal N., Surendranath A.R., Pathak J.L., Yu S., Chung C.Y. Bisphenol a (bpa) the mighty and the mutagenic. Toxicol Rep. 2018;5:76–84. doi: 10.1016/j.toxrep.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L., Suh B.I. Bisphenol a in dental materials: a review. JSM Dentistry. 2013;(1):1–5. [Google Scholar]
- 14.Kim W.B., Joshi U.A., Lee J.S. Making polycarbonates without employing phosgene: an overview on catalytic chemistry of intermediate and precursor syntheses for polycarbonate. Ind Eng Chem Res. 2004;43(9):1897–1914. doi: 10.1021/ie034004z. [DOI] [Google Scholar]
- 15.Kalachandra S., Kusy R. Comparison of water sorption by methacrylate and dimethacrylate monomers and their corresponding polymers. Polymer. 1991;32(13):2428–2434. doi: 10.1016/0032-3861(91)90085-W. [DOI] [Google Scholar]
- 16.Cook W.D. Thermal aspects of the kinetics of dimethacrylate photopolymerization. Polymer. 1992;33(10):2152–2161. doi: 10.1016/0032-3861(92)90882-W. [DOI] [Google Scholar]
- 17.Gajewski V.E., Pfeifer C.S., Fr ´oes-Salgado N.R., Boaro L.C., Braga R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz Dent J. 2012;23(5):508–514. doi: 10.1590/S010364402012000500007. [DOI] [PubMed] [Google Scholar]
- 18.Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci. 1997;105(2):97–116. doi: 10.1111/j.1600-0722.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 19.Sideridou I., Tserki V., Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23(8):1819–1829. doi: 10.1016/S0142-9612(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S.R., Patnaik A., Bhat I. Physical and thermo-mechanical characterizations of resin-based dental composite reinforced with silane-modified nanoalumina filler particle. Proc Inst Mech Eng Part L J Mater Des Appl. 2016;230(2):504–514. doi: 10.1177/1464420715581004. arXiv:https://doi.org/10.1177/1464420715581004. [DOI] [Google Scholar]
- 21.Barszczewska-Rybarek I., Jurczyk S. Comparative study of structure-property relationships in polymer networks based on bis-gma, tegdma and various urethane-dimethacrylates. Materials. 2015;8(3):1230–1248. doi: 10.3390/ma8031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karimzadeh A., Ayatollahi M.R., Bushroa A.R. Effect of dental restorative material type and shade oncharacteristics of two-layer dental composite systems. Lat Am J Solids Struct. 2016;13(10):1851–1865. doi: 10.1590/1679-78252562. [DOI] [Google Scholar]
- 23.Durner J., Schrickel K., Watts D.C., Ilie N. Determination of homologous distributions of Bis-EMA dimethacrylates in bulk-fill resin-composites by gc–ms. Dent Mater. 2015;31(4):473–480. doi: 10.1016/j.dental.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Montheard J.-P., Chatzopoulos M., Chappard D. 2-hydroxyethyl methacrylate (hema): chemical properties and applications in biomedical fields. J Macromol Sci Part C- Polym Rev. 1992;32(1):1–34. doi: 10.1080/15321799208018377. [DOI] [Google Scholar]
- 25.Wichterle O., LM D. Hydrophilic gels for biological use. Nature. 2016;185(4706):117–118. doi: 10.1038/185117a0. [DOI] [Google Scholar]
- 26.Refojo M.F., Yasuda H. Hydrogels from 2-hydroxyethyl methacrylate and propylene glycol monoacrylate. J Appl Polym Sci. 1965;9(7):2425–2435. doi: 10.1002/app.1965.070090707. [DOI] [Google Scholar]
- 27.Polydorou O., Knig A., Hellwig E., Kmmerer K. Uthethane dimethacrylate: a molecule that may cause confusion in dental research. J Biomed Mater Res Part B Appl Biomater. 2009;91B(1):1–4. doi: 10.1002/jbm.b.31383. [DOI] [PubMed] [Google Scholar]
- 28.Khatri C.A., Stansbury J.W., Schultheisz C.R., Antonucci J.M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent Mater. 2003;19:584–588. doi: 10.1016/S0109-5641(02)00108-2. [DOI] [PubMed] [Google Scholar]
- 29.Stansbury J.W., Dickens S.H. Network formation and compositional drift during photo-initiated copolymerization of dimethacrylate monomers. Polymer. 2001;42(15):6363–6369. [Google Scholar]
- 30.Alshali R.Z., Silikas N., Satterthwaite J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent Mater. 2013;29(9):e213–e217. doi: 10.1016/j.dental.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Landuyt K.L.V., Snauwaert J., Munck J.D., Peumans M., Yoshida Y., Poitevin A., et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28(26):3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 32.Hadis M.A., Shortall A.C., Palin W.M. Competitive light absorbers in photoactive dental resin-based materials. Dent Mater. 2012;28(8):831–841. doi: 10.1016/j.dental.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Pratap B., Gupta R.K. Evaluation of physical properties of silica filled resin based dental composites. Int J Eng Adv Technol. 2019;8(6):5047–5049. [Google Scholar]
- 34.Datar R.A., Rueggeberg F.A., Caughman G.B., Wataha J.C., Lewis J.B., Schuster G.S. Effects of sub-toxic concentrations of camphorquinone on cell lipid metabolism. J Biomater Sci Polym Ed. 2005;16(10):1293–1302. doi: 10.1163/156856205774269557. [DOI] [PubMed] [Google Scholar]
- 35.Park Y.-J., Chae K.-H., Rawls H. Development of a new photoinitiation system for dental light-cure composite resins. Dent Mater. 1999;15(2):120–127. doi: 10.1016/S0109-5641(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 36.Dunnick J.K., Brix A., Sanders J.M., Travlos G.S. N,n-dimethyl-p-toluidine, a component in dental materials, causes hematologic toxic and carcinogenic responses in rodent model systems. Toxicol Pathol. 2014;42(3):603–615. doi: 10.1177/0192623313489604. pMID: 23867143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge X., Ye Q., Song L., Laurence J.S., Spencer P. Synthesis and evaluation of a novel co-initiator for dentin adhesives: polymerization kinetics and leachables study. JOM. 2015;67(4):796–803. doi: 10.1007/s11837-015-1335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pratap B., Gupta R.K., Bhardwaj B., Nag M. Modeling based experimental investigation on polymerization shrinkage and micro-hardness of nano alumina filled resin based dental material. J Mech Behav Biomed Mater. 2019;99:86–92. doi: 10.1016/j.jmbbm.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 39.NOMOTO R., HIRASAWA T. Residual monomer and pendant methacryloyl group in light-cured composite resins. Dent Mater J. 1992;11(2):177–188. doi: 10.4012/dmj.11.177. [DOI] [PubMed] [Google Scholar]
- 40.Pratap B., Gupta R.K., Ghosh S.S., Bhardwaj B. Process parameter optimization for minimum polymerization shrinkage of resin based dental material. AIP Conference Proceedings. 2019 (Vol. 2148, No. 1, p. 030020). (September) AIP Publishing. [Google Scholar]
- 41.Dionysopoulos D., Tolidis K., Gerasimou P. The Effect of Composition,Temperature and Post-Irradiation Curing of Bulk Fill Resin Composites on Polymerization Efficiency. Mater Res. 2016;19(2):466–473. [Google Scholar]
- 42.Jeong T.-s., Kang H.-s., Kim S.-k., Kim S., Kim H.-i., Kwon Y.H. The effect of resin shades on microhardness, polymerization shrinkage, and color change of dental composite resins. Dent Mater J. 2009;28(4):438–445. doi: 10.4012/dmj.28.438. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer C.S., Ferracane J.L., Sakaguchi R.L., Braga R.R. Photopolymerization stress in dental composites. J Dent Res. 2008;87(11):1043–1047. doi: 10.1177/154405910808701114. [DOI] [PubMed] [Google Scholar]
- 44.Harahap K., Yudhit A., Sari F. In: Innovation in polymer science and Technology 2016 (IPST 2016), no. 223, IOP Conference series: materials science and engineering. Yudianti R., Azuma J., editors. 2017. Effect of bench time polymerization on depth of cure of dental composite resin; pp. 1–7. [DOI] [Google Scholar]
- 45.Muniz Gabrielle Ribeiro Lima, Souza Erick Miranda, Raposo Carolina Carramilo, Santana Ivone Lima. Influence of heat treatment on the sorption and solubility of direct composite resins. Indian J Dent Res. 2013;24(6):708–712. doi: 10.4103/0970-9290.127617. [DOI] [PubMed] [Google Scholar]
- 46.Shamszadeh S., Sheikh-al eslamian S.M., Hasani E., Abrandabadi A.N., Panahandeh N. Color stability of the bulk-fill composite resins with different thickness in response to coffee / water immersion. Int J Dent. 2016;2016:1–5. doi: 10.1155/2016/7186140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguiar Flvio Henrique Baggio, Lazzari Carolina Rodrigues, Loma D.A., Ambrosano A.M., Lovadino J.R. Effect of light curing tip distance and resin shade on microhardness of a hybrid resin composite. Braz Oral Res. 2005;19(4):302–306. doi: 10.1590/s1806-83242005000400012. [DOI] [PubMed] [Google Scholar]
- 48.Segal Pnina, Lugassy Diva, Mijiritsky Eitan, Dekel Michal, Ben-Amar Ariel, Ormianer Zeev, et al. The effect of the light intensity and light distances of LED and QTH curing devices on the hardness of two light-cured nano-resin composites. Mater Sci Appl. 2015;6(11):1071–1083. doi: 10.4236/msa.2015.611106. [DOI] [Google Scholar]
- 49.Palin W., Hadis M., Leprince J.G., Leloup G., Boland L., Fleming G.J.P., et al. Reduced polymerization stress of mapo-containing resin composites with increased curing speed, degree of conversion and mechanical properties. Dent Mater. 2014;30(5):507–516. doi: 10.1016/j.dental.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Mousavinasab S.M., Barekatain M., Sadeghi E., Nourbakhshian F. Evaluation of light curing distance and mylar strips color on surface hardness of two different dental composite resins. Open Dent J. 2014;8:144–147. doi: 10.2174/1874210601408010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferdous S.F., Sarker M.F., Adnan A. Role of nanoparticle dispersion and filler-matrix interface on the matrix dominated failure of rigid c60-pe nanocomposites: a molecular dynamics simulation study. Polymer. 2013;54(10):2565–2576. doi: 10.1016/j.polymer.2013.03.014. [DOI] [Google Scholar]
- 52.Sugerman S.J.M.G. Kenrich Petrochemicals; Bkayonne, N.J: 1993. Ken-react reference manual: titanate, zirconate and aluminate coupling agents. [Google Scholar]
- 53.Cheng H., Tsoi J.-H., Zwahlen R., Matinlinna J. Effects of silica-coating and a zirconate coupling agent on shear bond strength of flowable resinzirconia bonding. Int J Adhes Adhes. 2014;50:11–16. doi: 10.1016/j.ijadhadh.2013.12.025. [DOI] [Google Scholar]
- 54.Bose S., Mahanwar P.A. Effect of titanate coupling agent on the mechanical, thermal, dielectric, rheological, and morphological properties of filled nylon 6. J Appl Polym Sci. 2005;99(1):266–272. doi: 10.1002/app.22472. [DOI] [Google Scholar]
- 55.Nihei T. Dental applications for silane coupling agents. J Oral Sci. 2014;58(2):151–155. doi: 10.2334/josnusd.16-0035. [DOI] [PubMed] [Google Scholar]
- 56.Kumar S.R., Patnaik A., Bhat I.K. Physical and thermo-mechanical characterizations of resin-based dental composite reinforced with Silane-Modified nanoalumina filler particle. Proc Inst Mech Eng Part L J Mater Des Appl. 2016;230(2):504–514. doi: 10.1177/1464420715581004. [DOI] [Google Scholar]
- 57.Antonucci J., Dickens S., Fowler B., Xu H. J Res Natl Inst Stand Technol. 2005;110(5):541. doi: 10.6028/jres.110.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishiyama N., Komatsu K., Fukai K., Nemoto K., Kumagai M. Influence of adsorption characteristics of silane on the hydrolytic stability of silane at the silica-matrix interface. Composites. 1995;26(4):309–313. doi: 10.1016/0010-4361(95)93674-9. [DOI] [Google Scholar]
- 59.CRAIG R., DOOTZ E. Effect of mixed silanes on the hydrolytic stability of composites. J Oral Rehabil. 1996;23(11):751–756. doi: 10.1046/j.1365-2842.1996.d01-194.x. [DOI] [PubMed] [Google Scholar]
- 60.Arsecularatne J., Chung N., Hoffman M. An in vitro study of the wear behaviour of dental composites. Biosurface Biotribology. 2016;2(3):102–113. doi: 10.1016/j.bsbt.2016.09.002. [DOI] [Google Scholar]
- 61.Heintze S.D., Forjanic M., Ohmiti K., Rousson V. Surface deterioration of dental materials after simulated toothbrushing in relation to brushing time and load. Dent Mater. 2010;26:306–319. doi: 10.1016/j.dental.2009.11.152. [DOI] [PubMed] [Google Scholar]
- 62.Addy M., Shellis R. Interaction between attrition,Abrasion and Erosion in tooth wear. Dental Erosion. 2006;20:17–31. doi: 10.1159/000093348. [DOI] [PubMed] [Google Scholar]
- 63.Sonal M.G., Kumar Shiv Ranjan, Patnaik Amar, Meena Anoj. Effect of adding nanosilica particulate filler on the wear behavior of dental composite. Polym Compos. 2017 [Google Scholar]
- 64.Gan X.Q., Cai Z.B., Zhang B.R., Zhou X.D., Yu H.Y. Friction and wear behaviors of indirect dental restorative composites. Tribol Lett. 2012;46:75–86. [Google Scholar]
- 65.Hu X., Zhang Q., Ning J., Wu W., Li C. Study of two-body wear performance of dental materials. J Natl Med Assoc. 2017:1–6. doi: 10.1016/j.jnma.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Chadda H., Satapathy B.K., Patnaik A., Ray A.R. Mechanistic interpretations of fracture toughness and correlations to wear behavior of hydroxyapatite and silica/hydroxyapatite filled bis-GMA/TEGDMA micro/hybrid dental restorative composites. Compos. Part B Eng. 2017;130:132–146. doi: 10.1016/j.compositesb.2017.07.069. [DOI] [Google Scholar]
- 67.Altaie A., Bubb N.L., Franklin P., Dowling A.H., Fleming G.J.P., Wood D.J. An approach to understanding tribological behaviour of dental composites through volumetric wear loss and wear mechanism determination; beyond material ranking. J Dent. 2017;59:41–47. doi: 10.1016/j.jdent.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Souza J.C.M., Bentes A.C., Reis K., Gavinha S., Buciumeanu M., Henriques B., et al. Abrasive and sliding wear of resin composites for dental restorations. Tribol Int. 2016;102:154–160. doi: 10.1016/j.triboint.2016.05.035. [DOI] [Google Scholar]
- 69.Zhi L., Bortolotto T., Krejci I. Comparative in vitro wear resistance of CAD/CAM composite resin and ceramic materials. J Prosthet Dent. 2016;115:199–202. doi: 10.1016/j.prosdent.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Morozova Y., Holik P., Ctvrtlik R., Tomastik J., Azar B., Sedlat Jurskov E., et al. Methods of wear measuring in dentistry. Iosr J Dent Med Sci. 2016;15(6):63–68. [Google Scholar]
- 71.Mjor I.A., Moorhead J.E., Dahl J.E. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi R.L. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Van Nieuwenhuysen J.P., D’Hoore W., Carvalho J., Qvist V. Long-term evaluation of extensive restorations in permanent teeth. J Dent. 2003;31:395–405. doi: 10.1016/s0300-5712(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 74.Baran G.R., Boberick K.G., McCool J.I. Fatigue of restorative materials. Crit Rev Oral Biol Med. 2001;12:350–360. doi: 10.1177/10454411010120040501. [DOI] [PubMed] [Google Scholar]
- 75.White S.R., Sottos N.R., Geubelle P.H., Moore J.S., Kessler M.R., Sriram S.R., et al. Autonomic healing of polymer composites. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 76.Wertzberger B.E., Steere J.T., Pfeifer R.M., Nensel M.A., Latta M.A., Gross S.M. Physical characterization of a self-healing dental restorative material. J Appl Polym Sci. 2010;118:428–434. [Google Scholar]
- 77.Then S., Neon G.S., kasim N.H. Performance of melamine modified urea–formaldehyde microcapsules in a dental host material. J Appl Polym Sci. 2011;122:2557–2562. [Google Scholar]
- 78.Bevan C., Snellings W.M., Dodd D.E., Egan G.F. Subchronic toxicity study of dicyclopentadiene vapor in rats. Toxicol Ind Health. 1992;8:353–367. [PubMed] [Google Scholar]
- 79.Caruso M.M., Delafuente D.A., Ho V., Sottos N.R., Moore J.S., White S.R. Solvent-promoted self-healing epoxy materials. Macromolecules. 2007;40:8830–8832. [Google Scholar]
- 80.Ouyang X., Huang X., Pan Q., Zuo C., Huang C., Yang X., et al. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. J Dent. 2011;39:825–833. doi: 10.1016/j.jdent.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Wu J., Weir M.D., Zhang Q., Zhou C., Melo M.A., Xu H.H. Novel self-healing dental resin with microcapsules of polymerizable triethylene glycol dimethacrylate and N, N-dihydroxyethyl-p-toluidine. Dent Mater. 2016;32(Feb (2)):294–304. doi: 10.1016/j.dental.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Q., Mishra A.K., Kim N.H., Kuila T., Lau K.T., Lee J.H. Effects of processing conditions of poly (methylmethacrylate) encapsulated liquid curing agent on the properties of self-healing composites. Compos Part B Eng. 2013;49(Jun):6–15. [Google Scholar]
- 83.Wu J., Weir M.D., Melo M.A., Strassler H.E., Xu H.H. Effects of water-aging on self-healing dental composite containing microcapsules. J Dent. 2016;47(Apr):86–93. doi: 10.1016/j.jdent.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen C., Wu J., Weir M., Wang L., Zhou X., Xu H., et al. Dental composite formulation design with bioactivity on protein adsorption combined with crack-healing capability. J Funct Biomater. 2017;8(Sep (3)):40. doi: 10.3390/jfb8030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang N., Zhang K., Xie X., Dai Z., Zhao Z., Imazato S., et al. Nanostructured polymeric materials with protein-repellent and anti-caries properties for dental applications. Nanomaterials. 2018;8(Jun (6)):393. doi: 10.3390/nano8060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yahyazadehfar M., Huyang G., Wang X., Fan Y., Arola D., Sun J. Durability of self-healing dental composites: a comparison of performance under monotonic and cyclic loading. Mater Sci Eng C. 2018;93(Dec):1020–1026. doi: 10.1016/j.msec.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huyang G., Sun J. Clinically applicable self-healing dental resin composites. MRS Adv. 2016;1(8):547–552. [Google Scholar]
- 88.Huyang G., Debertin A.E., Sun J. Design and development of self-healing dental composites. Mater Des. 2016;94:295–302. doi: 10.1016/j.matdes.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gilabert F.A., Garoz D., Van Paepegem W. Stress concentrations and bonding strength in encapsulation-based self-healing materials. Mater Des. 2015;67:28–41. [Google Scholar]
- 90.Yue S., Wu J., Zhang Q., Zhang K., Weir M.D., Imazato S., et al. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. J Dent. 2018;75:48–57. doi: 10.1016/j.jdent.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 91.DEMARCO F.F., COLLARES K., CORREA M.B., CENCI M.S., de MORAES R.R., OPDAM N.J. Should my composite last forever? Why are they failing? Braz Oral Res. 2017;31(suppl 1):92–99. doi: 10.1590/1807-3107bor-2017.vol31.0056. [DOI] [PubMed] [Google Scholar]
- 92.Imazato S., Ehara A., Torii M., Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26(3):267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 93.Imazato S., Kinomoto Y., Tarumi H., Ebisu S., Tay F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003;19(4):313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 94.Miki S., Kitagawa H., Kitagawa R., Kiba W., Hayashi M., Imazato S. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent Mater. 2016;32(9):1095–1102. doi: 10.1016/j.dental.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J.F., Wu R., Fan Y., Liao S., Wang Y., Wen Z.T., et al. Antibacterial dental composites with chlorhexidine and mesoporous silica. J Dent Res. 2014;93(12):1283–1289. doi: 10.1177/0022034514555143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stencel R., Kasperski J., Pakie la W., Mertas A., Bobela E., Barszczewska-Rybarek I., et al. Properties of experimental dental composites containing antibacterial silver-releasing filler. Materials. 2018;11(6):1031. doi: 10.3390/ma11061031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imazato S., hua Chen J., Ma S., Izutani N., Li F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn Dent Sci Rev. 2012;48(2):115–125. doi: 10.1016/j.jdsr.2012.02.003. [DOI] [Google Scholar]
- 98.Imazato S., Torii M., Tsuchitani Y., McCabe J.F., Russell R.R. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 99.Imazato S., Ohmori K., Russell R.R., McCabe J.F., Momoi Y., Maeda N. Determination of bactericidal activity of antibacterial monomer MDPB by a viability staining method. Dent Mater J. 2008;27:145–148. doi: 10.4012/dmj.27.145. [DOI] [PubMed] [Google Scholar]
- 100.Imazato S., Ebi N., Tarumi H., Russell R.R., Kaneko T., Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20:899–903. doi: 10.1016/s0142-9612(98)00247-6. 20. [DOI] [PubMed] [Google Scholar]
- 101.Imazato S., Torii Y., Takatsuka T., Inoue K., Ebi N., Ebisu S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. J Oral Rehabil. 2001;28:314–319. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 102.Beyth N., Yudovin-farber I., Bahir R., Domb A.J., Weiss E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Cheng L., Zhang K., Weir M.D., Melo M.A.S., Zhou X., Xu H.H. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine. 2015;10(4):627–641. doi: 10.2217/nnm.14.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y., Samoei G.K., Lallier T.E., Xu X. Synthesis and characterization of new antibacterial fluoride-releasing monomer and dental composite. ACS Macro Lett. 2012;2(1):59–62. doi: 10.1021/mz300579y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delaviz Y., Liu T.W., Deonarain A.R., Finer Y., Shokati B., Santerre J.P. Physical properties and cytotoxicity of antimicrobial dental resin adhesives containing dimethacrylate oligomers of Ciprofloxacin and Metronidazole. Dent Mater. 2019;35(2):229–243. doi: 10.1016/j.dental.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 106.Chen H., Wang R., Zhang J., Hua H., Zhu M. Synthesis of core-shell structured ZnO@ m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent Mater. 2018;34(12):1846–1855. doi: 10.1016/j.dental.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 107.Boaro L.C.C., Campos L.M., Varca G.H.C., dos Santos T.M.R., Marques P.A., Sugii M.M., et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent Mater. 2019 doi: 10.1016/j.dental.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Imazato S., Ebi N., Takahashi Y., Kaneko T., Ebisu S., Russell R.R. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–3609. doi: 10.1016/s0142-9612(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 109.Imazato S., Imai T., Russell R.R., Torii M., Ebisu S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J Biomed Mater Res. 1998;39:511–515. doi: 10.1002/(sici)1097-4636(19980315)39:4<511::aid-jbm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 110.Pratap B., Gupta R.K., Bhardwaj B. Advances in industrial and production engineering. Springer; Singapore: 2019. Study of sliding wear behavior of alumina oxide filled fiber composite using design of experiment; pp. 735–742. [Google Scholar]